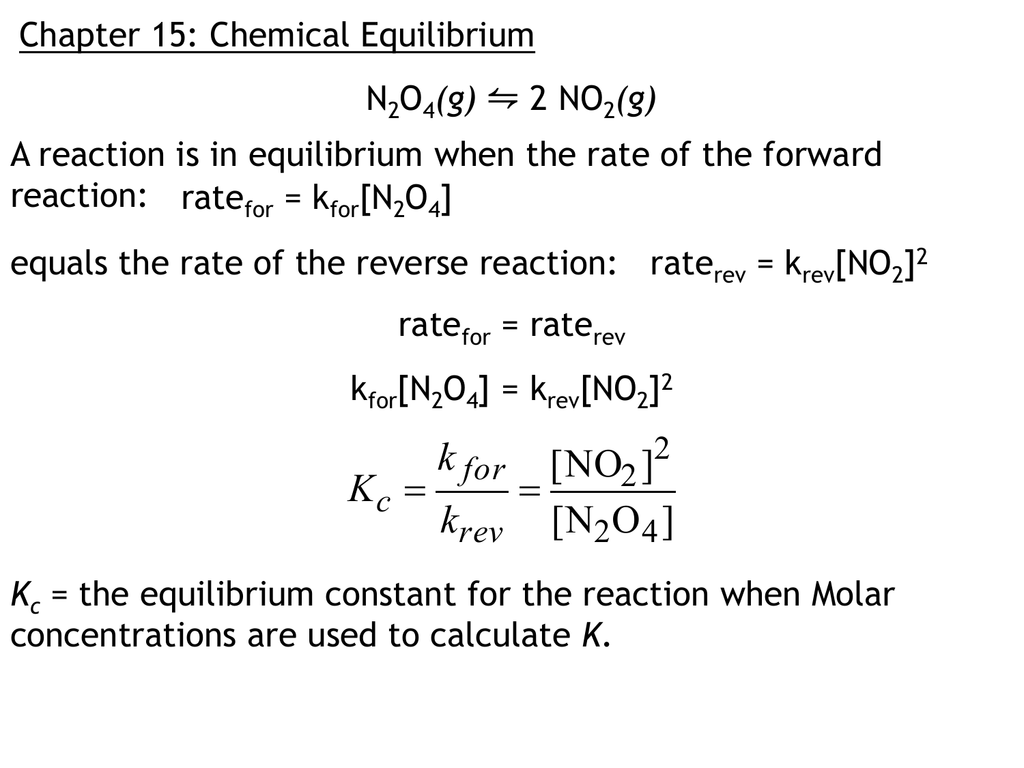
A state of dynamic equilibrium is a situation in which the concentrations of reactants and products in a chemical reaction remain constant over time, despite the fact that the reaction itself is ongoing. In other words, the rate of the forward reaction is equal to the rate of the reverse reaction.
One example of a system in dynamic equilibrium is the dissociation of ag2co3, or silver carbonate, into silver ions (Ag+) and carbonate ions (CO32-). This reaction can be represented by the following equation:
Ag2CO3 (s) -> 2Ag+ (aq) + CO32- (aq)
At a given temperature and pressure, the forward reaction (dissociation) and the reverse reaction (recombination) will occur at the same rate, leading to a state of dynamic equilibrium. This means that the concentrations of Ag+ and CO32- in solution will remain constant, even though the reaction is still occurring.
Dynamic equilibrium is an important concept in chemistry, as it allows us to predict the behavior of chemical systems under different conditions. By understanding how the concentration of reactants and products changes over time, we can better predict the outcomes of chemical reactions and make more informed decisions in a variety of fields, including medicine, manufacturing, and environmental science.
In the case of ag2co3, understanding the state of dynamic equilibrium can be useful in a number of applications. For example, silver carbonate is often used as a catalyst in the production of chemicals, and understanding the equilibrium state of the dissociation reaction can help chemists optimize the conditions of the reaction for maximum efficiency. Similarly, understanding the equilibrium state of ag2co3 can help us predict the behavior of silver ions in the environment, which can have important implications for environmental health and safety.
Overall, the concept of dynamic equilibrium is an important one in chemistry, and understanding it allows us to make more informed decisions about the behavior of chemical systems. By examining the equilibrium state of ag2co3 and other chemical reactions, we can better understand the underlying principles of chemistry and use that knowledge to improve our world.