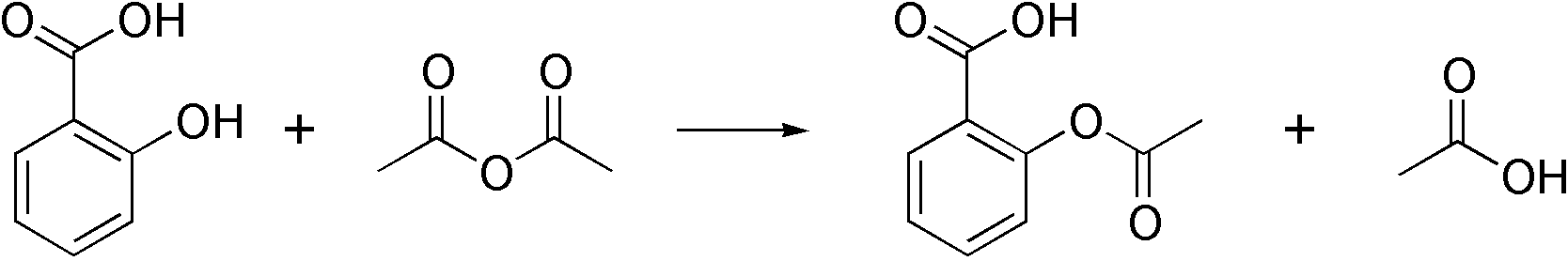
Acetylsalicylic acid, more commonly known as aspirin, is a widely used pain reliever and anti-inflammatory agent. It was first synthesized by the German chemist Felix Hoffmann in 1897, and has since become an integral part of many households around the world. The synthesis of aspirin involves the acetylation of salicylic acid, a compound that occurs naturally in plants such as willow bark and meadowsweet.
The synthesis of aspirin begins with the conversion of salicylic acid into its sodium salt, which is more reactive and easier to work with. This is typically done by reacting salicylic acid with sodium hydroxide in an aqueous solution. The resulting sodium salicylate can then be acetylated using acetic anhydride as the acetylating agent.
The acetylation reaction is typically carried out in the presence of a small amount of an acidic catalyst, such as sulfuric acid or hydrochloric acid, to facilitate the transfer of the acetyl group from acetic anhydride to the salicylic acid. This reaction proceeds via a nucleophilic substitution mechanism, in which the acetyl group of the acetic anhydride acts as a nucleophile and attacks the electrophilic carbon atom of the salicylic acid.
The acetylated product, known as acetylsalicylic acid, can then be isolated from the reaction mixture by techniques such as crystallization or filtration. It is typically purified by recrystallization to remove any impurities or contaminants that may be present.
The synthesis of aspirin is a simple and efficient process that has been widely used for over a century. In addition to its use as a pain reliever and anti-inflammatory agent, aspirin has also been shown to have beneficial effects on the cardiovascular system, making it a popular choice for preventing heart attacks and strokes. Overall, the synthesis of acetylsalicylic acid has had a significant impact on the field of medicine and has greatly improved the quality of life for many people around the world.
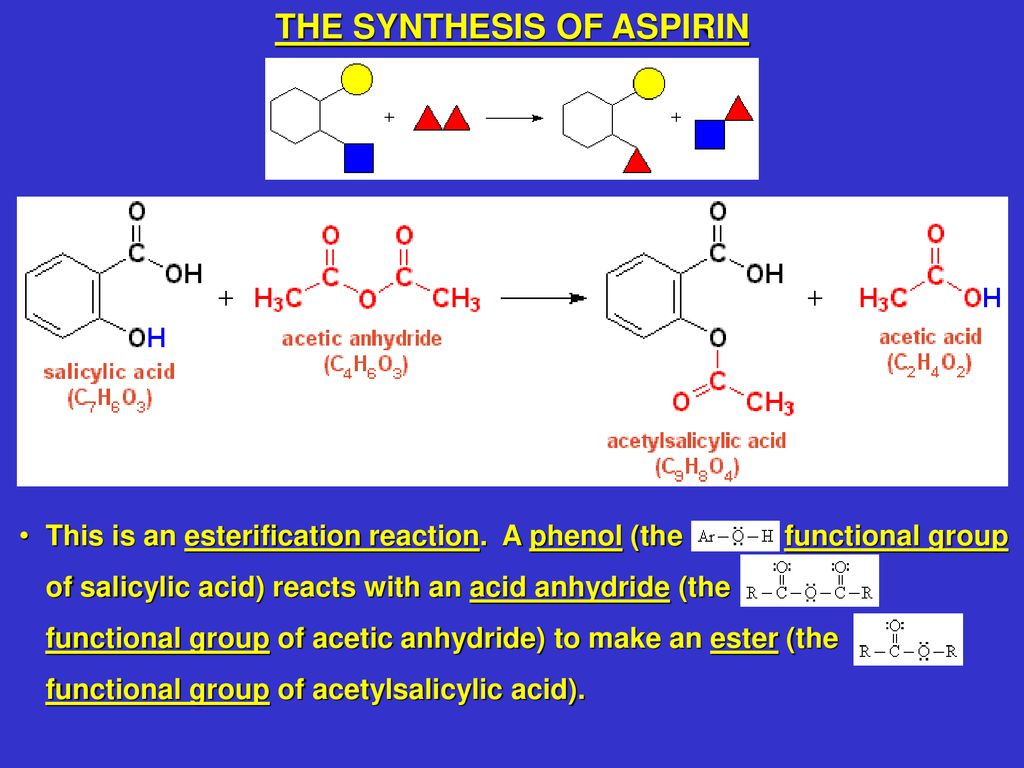
Synthesis of Acetylsalicylic Acid

The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid. This result could have been due to errors when carrying out the procedure in the lab as mentioned before. If the reaction has not fully completed then the Aspirin formed would not be pure, as it would contains unreacted reactants. Further, more molecules can bind to the remaining free substituents on these molecules to create a macromolecule, or polymer. As the temperature of the water increases, the solubility of aspirin increases due to an increased amount of interactions and collisions. Nonacetylated salicylates are also used in medical practice.
Acetylsalicylic Acid
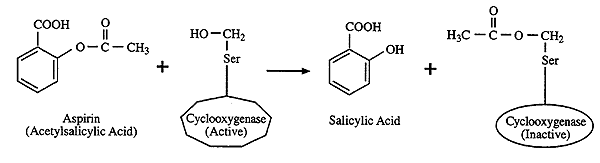
It is believed that the primary mechanism of action is the irreversible acetylation of cyclooxygenase, which results in the inability to synthesize prostaglandins, prostacyclins, and thromboxane. The information on how aspirin is carried out in industry will be established using various text books and internet sources that will be referenced at a later date. Discussion and Interpretation Analysing the results obtained it is clear that the experiment was a success. It contains the product in solution. Salicylic acid can be used to create two different types of esters. When the aspirin enters the stomach, some of it absorbs within the stomach.
Victoriana Magazine
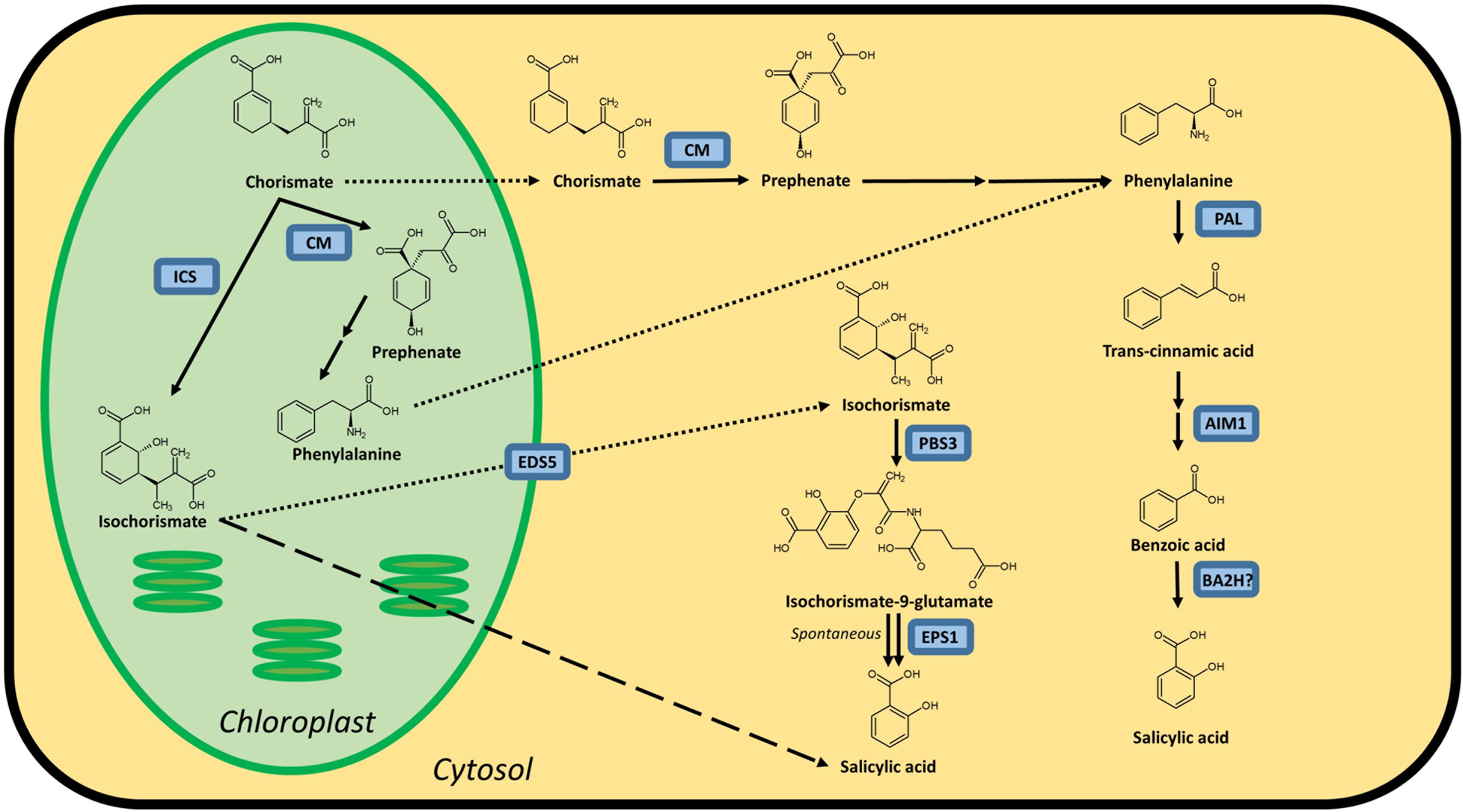
In vivo, ASA is rapidly hydrolyzed by unspecific esterases of the plasma to the also pharmacologically active main metabolite salicylic acid SA, Fig. Carefully, and slowly, pour the filtrate into the acid mixture while stirring. Victoriana showcases Victorian style home décor and furniture, Victorian clothing and accessories, Victorian weddings and Christmas. High doses of aspirin given to mice on day 6 of gestation produced large incidence of lethal deformities. The Merck Index, which is an encyclopedia of chemicals, drugs and biologicals, lists the following information under aspirin: acetylsalicylic acid; monoclinic tablets or needle-like crystals; mp 135 °C rapid heating ; is odorless, but in moist air it is gradually hydrolyzed into salicylic and acetic acids; one gram dissolves in 300 mL of water at 25 °C, in 100 mL of water at 37 °C, in 5 mL alcohol, in 17 mL chloroform.
Synthesis and Purification of Acetylsalicylic Acid
Dispose of filtrate in the appropriately labeled waste jar. Aspirin is a weak acid and it tends to ionize give up a H atom in an aqueous medium at high pH. Therefore precautions to chemicals such as Acetylsalicylic acid were taken as it is irritating to the eyes. After addition of the seed, the crystallization started with feeding the antisolvent, water into the solution at a rate of 2. Under room temperature conditions, it would take nearly a month for all of the aspirin to be degraded. Victoriana divides the 19th century into categories such as Victorian Weddings, Victorian Clothing, Victorian décor, Victorian Architecture, Victorian Houses, plus more; everything needed for Victorian era lifestyle, decorating and restoration.
Synthesis of Acetyl Salicylic Acid (Aspirin) Essay Example

The factors that contributed to the inaccuracy of the experiment are the following: Reflux: The apparatus used in the experiment were quite old. Dose as in normal renal function Hepatic Dose : Mild to moderate hepatic impairment: Liver function tests should be monitored. Dispose of solid in the appropriately labeled waste jar, and discard the filter paper. Aspirin is widely used for head and neuralgic pains, rheumatic conditions, painful symptoms of various etiologies, and eliminating painful feelings during menstruation. Human Chronic salicylism presents similarly to acute toxicity, although more severe symptoms may be present at lower serum concentrations. Balance:The balance used to calculate the mass of the wet and dry product along with the beaker was considerably outdated.
Acetylsalicylic
Acetylsalicylic acid may be partially hydrolyzed; this, plus the easily noticeable smell of acetic acid, can be recognized by a trial with FeCl 3 and observing whether the violet color of FeCl 3 is produced. Wait and observe a little longer until all of the aspirin has melted. As the kinetic energy increase, molecules move faster and collide with other molecules, therefore there are more interactions. If necessary, gently scratch the bottom of the flask with a glass rod to initiate crystal formation on microscopic glass particles. Which of the atoms labeled 1-4 is an electrophile? Salicylic acid is removed during the purification steps as well.