The Aldol Condensation is a chemical reaction that involves the condensation of two molecules of aldehyde or ketone, resulting in the formation of a molecule with a carbon-carbon bond and the release of a small molecule such as water or alcohol. This reaction is an important process in organic chemistry, as it allows for the synthesis of a wide range of compounds, including many that are biologically active.
The Aldol Condensation can be carried out under a variety of conditions, depending on the specific reactants and the desired outcome of the reaction. One common method for performing the Aldol Condensation involves the use of an aqueous solution of a base, such as sodium hydroxide or potassium hydroxide, to facilitate the formation of the carbon-carbon bond. The reactants are typically dissolved in a solvent, such as ethanol or acetone, and the reaction is allowed to proceed at room temperature or at an elevated temperature.
One key feature of the Aldol Condensation is the formation of a carbon-carbon bond, which is important for the synthesis of many complex molecules. In addition, the small molecule that is released during the reaction, such as water or alcohol, can be easily removed, allowing for the synthesis of pure, isolated products.
There are several variations of the Aldol Condensation, including the crossed Aldol Condensation, in which the reactants are not the same, and the Knoevenagel Condensation, in which the reactants are not aldehydes or ketones. These variations allow for the synthesis of a wider range of compounds, making the Aldol Condensation a valuable tool for the synthesis of complex molecules.
In conclusion, the Aldol Condensation is a powerful chemical reaction that allows for the synthesis of a wide range of compounds through the condensation of aldehydes or ketones. Its versatility and effectiveness make it an important tool in the field of organic chemistry.
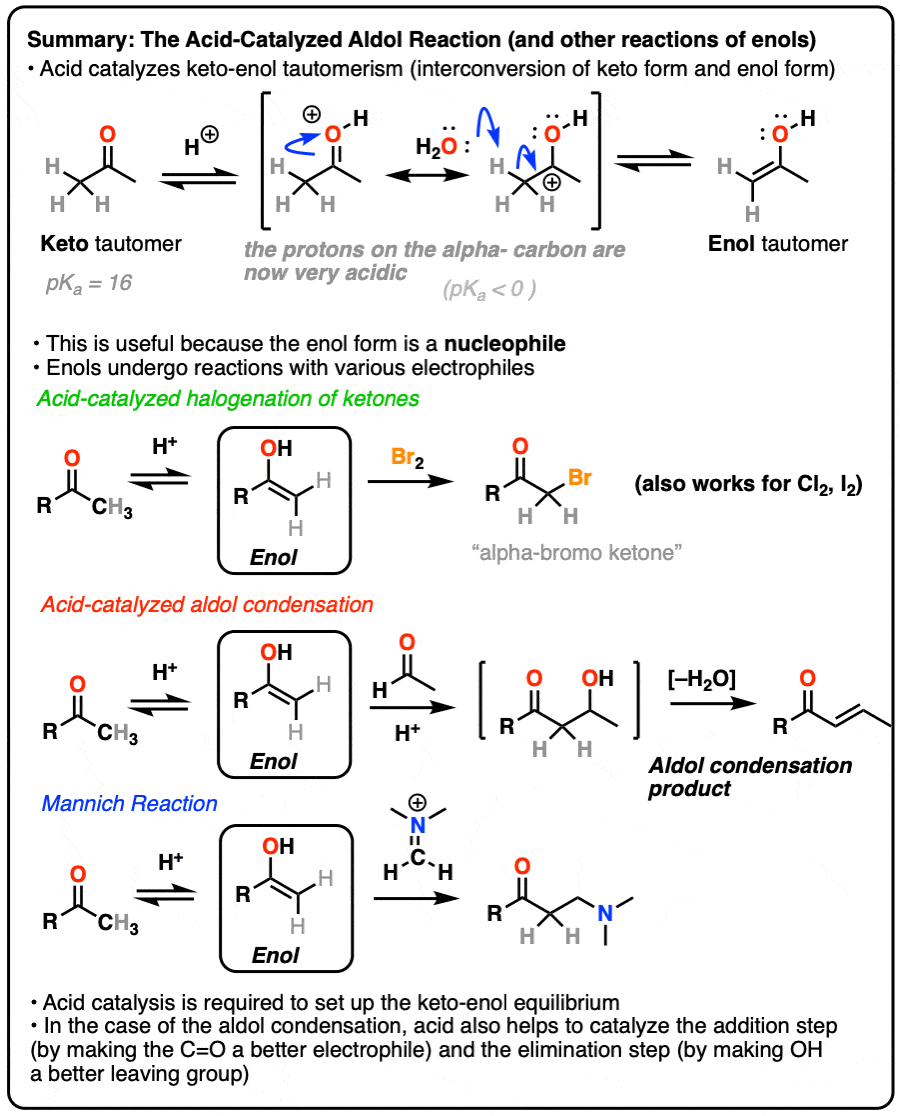
23.8: The Aldol Reaction and Condensation of Ketones and Aldehydes

In order to carry out the experiment, a Mel-temp apparatus, as well as vacuum distillation, was performed. Conclusion In this aldol condensation, 1 mole of acetone and 2 moles of benzaldehyde were reacted with each other to produce dibenzalacetone in the presence of NaOH. This alpha hydrogen is acidic because of its Multistep Synthesis Chemistry Experiment Tetraphenylcyclopentadienone In this laboratory experiment a synthesis was performed through several separate steps. After dissolving, crystals should reform as soon as the solution cools to room temperature without the aid of ice. An acid-base reaction; the alkoxide deprotonates a water molecule creating hydroxide and the β-hydroxyaldehydes or aldol product. As with other aldol reaction the addition of heat causes an aldol condensation to occur.
Aldol Condensation
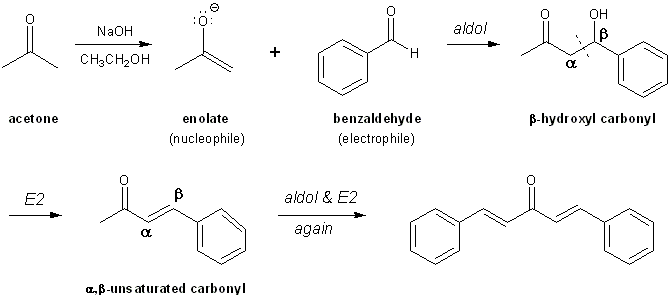
In a glass vial, 0. However, as a last resort, you could draw off the basic aqueous layer is this the upper or lower layer? Williamson and Katherine M. Addressing the fundamental aspects of the protein will allow us to explore the potential applications and implications of the protein if it has been modified. Combine the benzaldehyde 2. The reaction also showed the importance of an enolate and the role it played in the mechanism. Remove the tube from the hot sand bath, remove the boiling stick, and place the tube in an insulated container to cool slowly to room temperature. Collect the crystallized solid using filtration using a B? The benzalacetone, once formed, can then easily react with another mole of benzaldehyde to give the product, dibenzalacetone.
Aldol Condensation Experiment blog.sigma-systems.com

Wash the precipitate with a small amount of water. An alpha, beta, and unsaturated ketone are synthesized using solvent-free conditions as well as purifying the product through re-crystallization and to identify and analyze purity through melting point analysis. The amount of toluene required given the hypothetical situation would be 16 mL. Overall the general reaction involves a dehydration of an aldol product to form an alkene: Figure: General reaction for an aldol condensation Going from reactants to products simply Figure: The aldol condensatio example Aldol Condensation Acid Catalyzed Mechanism Under acidic conditions an enol is formed and the hydroxy group is protonated. Conjugation of the newly formed double bond with the carbonyl group or of the benzene ring, as shown below stabilizes the unsaturated product and provides the thermodynamic driving force for the dehydration process. Second, aldehydes lacking alpha-hydrogens can only function as acceptor reactants, and this reduces the number of possible products by half.