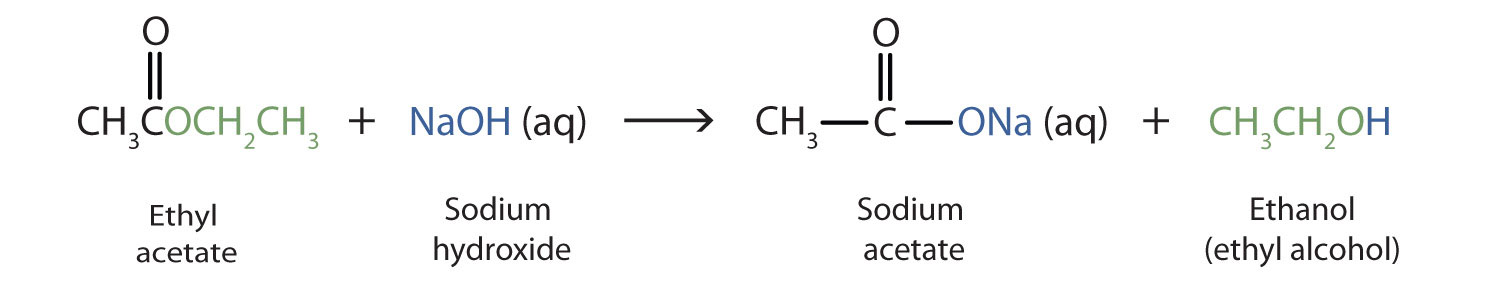
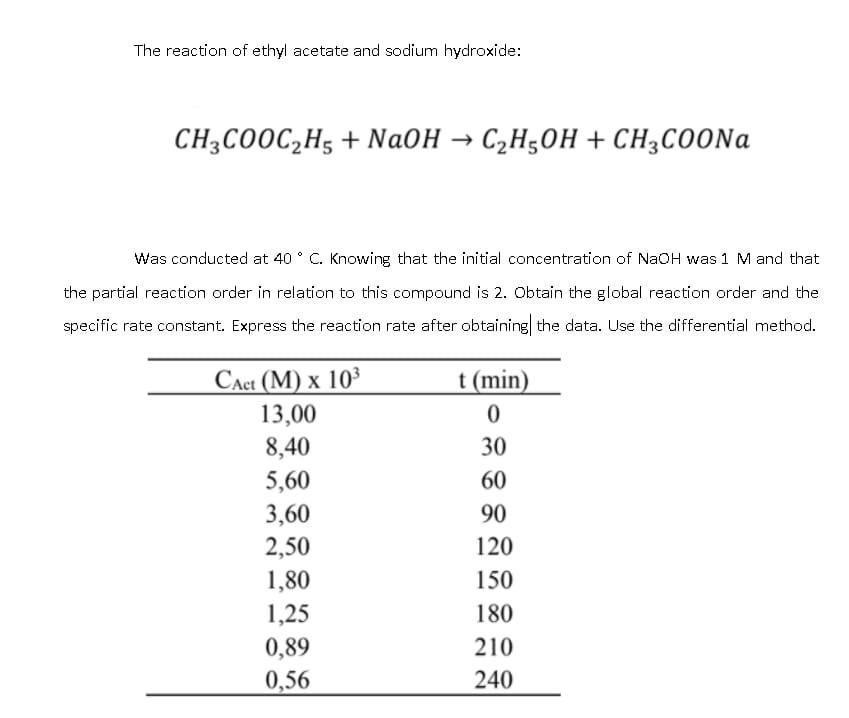
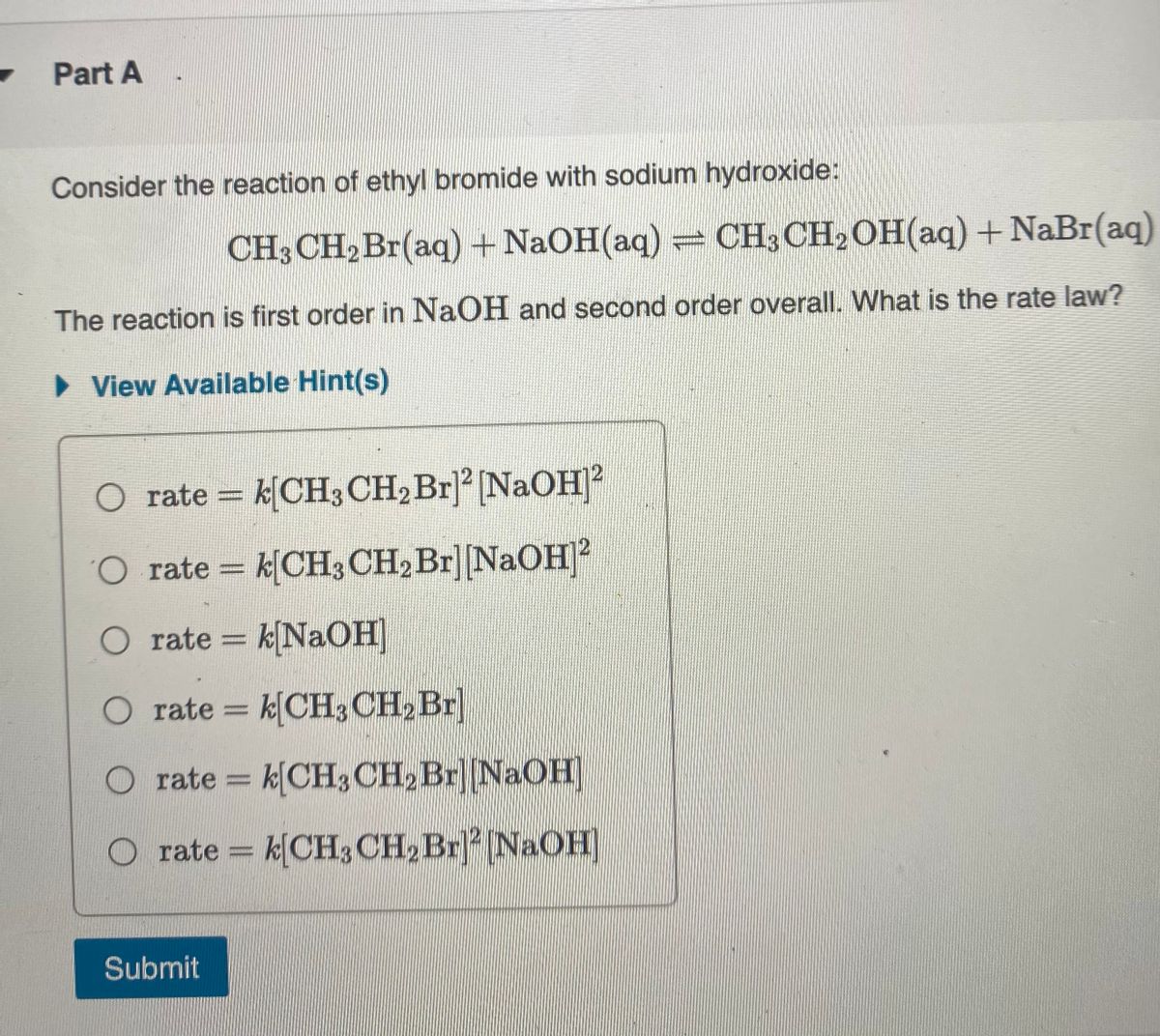
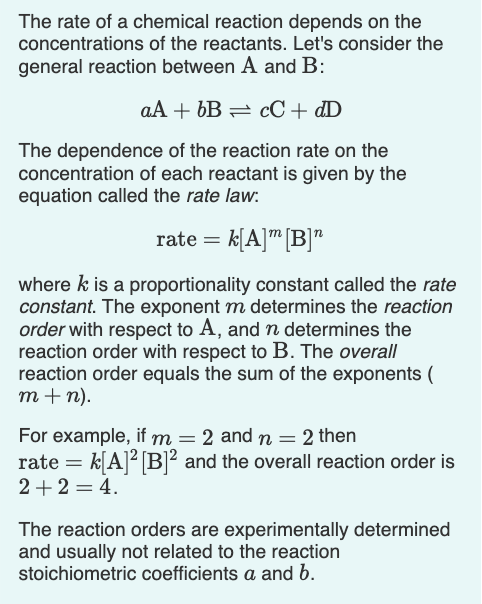
Ethyl acetate, also known as CH3COOC2H5 or acetate ester, is a common solvent used in various industries including pharmaceuticals, perfumes, and flavorings. It is a colorless liquid with a fruity, sweet odor, and is chemically classified as an ester.
Ethyl acetate is produced through the esterification of acetic acid and ethanol. The reaction between these two compounds is catalyzed by an acid catalyst, such as sulfuric acid or hydrochloric acid. The resulting product is a mixture of ethyl acetate and water, which can be separated through distillation.
One common use of ethyl acetate is as a solvent in the production of paints and coatings. It is also used as a solvent in the production of inks, adhesives, and cleaning agents. In the pharmaceutical industry, it is often used as a solvent for drug formulations and as a flavoring agent in medications. In the food and beverage industry, ethyl acetate is used as a flavor and fragrance ingredient in products such as fruit juices, soft drinks, and liqueurs.
Sodium hydroxide, also known as NaOH or caustic soda, is a strong base commonly used in the manufacturing of soaps, detergents, and textiles. It is a white, hygroscopic solid that is highly soluble in water and produces heat when dissolved. Sodium hydroxide is also used as a drain cleaner, as it can dissolve grease and oils that may clog pipes.
Sodium hydroxide is produced through the electrolysis of brine, which is a solution of sodium chloride (common table salt) in water. The process involves passing an electric current through the brine, causing the sodium and chloride ions to separate and form solid sodium hydroxide and chlorine gas.
In the chemical industry, sodium hydroxide is used as a strong base in the production of a wide range of products including paper, detergents, soaps, and textiles. It is also used in the refining of petroleum products and in the production of biodiesel. In the food industry, it is used to adjust the pH of food products and as a food additive to improve texture and stability.
In conclusion, ethyl acetate and sodium hydroxide are both important chemicals with a wide range of applications in various industries. Ethyl acetate is a common solvent used in the production of paints, adhesives, and cleaning agents, as well as in the pharmaceutical and food and beverage industries. Sodium hydroxide, on the other hand, is a strong base used in the manufacturing of soaps, detergents, and textiles, as well as in the refining of petroleum products and in the production of biodiesel.