Cis-norbornene 5,6-endo-dicarboxylic anhydride, also known as ENDO-CBA or simply ENDO, is a chemical compound that belongs to the class of organic compounds known as norbornenes. It is an anhydride, meaning that it is a compound formed from the dehydration of a dicarboxylic acid.
ENDO is a colorless, crystalline solid at room temperature and has a pungent, acrid smell. It is highly reactive and can be easily hydrolyzed, or broken down, in the presence of water.
ENDO has a number of important industrial uses. It is used as an intermediate in the synthesis of polymers and other chemicals, and it has been used in the production of resins, fibers, and adhesives. It is also used in the manufacture of pharmaceuticals, pesticides, and dyes.
ENDO is synthesized through the cycloaddition of norbornadiene with maleic anhydride. This reaction takes place at high temperatures and pressures, and the resulting compound is then purified and crystallized.
Despite its useful properties, ENDO can be hazardous to handle and can cause serious health effects if inhaled or ingested. It can irritate the eyes, skin, and respiratory system, and can cause coughing, sneezing, and difficulty breathing. It is classified as a flammable liquid and should be handled with caution.
In conclusion, cis-norbornene 5,6-endo-dicarboxylic anhydride is a versatile chemical compound with a range of industrial uses, but it must be handled with caution due to its potential health and safety risks.
129

There are other metal hydrides used in the reduction of carbonyl groups such as lithium aluminum hydride LiAlH4. The percent yield was satisfactory, having a 68% yield. In order to un- dimerize dicyclopentadiene, it must be heated to just under its boiling point to make fresh cyclopentadiene. The chemicals used are Maleic anhydride and Cyclopentadiene. The starting compound could have been one of four alcohols, cyclopentanol, cyclohexanol, 3-heptanol, or 2-heptanol.
cis

Since these were the only four initial compounds, the ketone obtained at the end of the experiment could only be one of four products, cyclopentanone, cyclohexanone, 3-heptanone, or 2-heptanone. The key to making this discovery was the melting point and TLC results! I believe that we did accomplish what we set out to do in this lab. Didier Villemin; Bouchta Labiad; Andre Loupy. The Diels-Alder reaction has high synthetic utility for making unsaturated 6-membered rings Kahn, 2011. To optimize this yield, consider the steps in how the reagents are introduced to the reaction mixture in terms. GHS Hazard and Precautionary Statements Hazard Statements: H317-H318-H334 May cause an allergic skin reaction. .
6
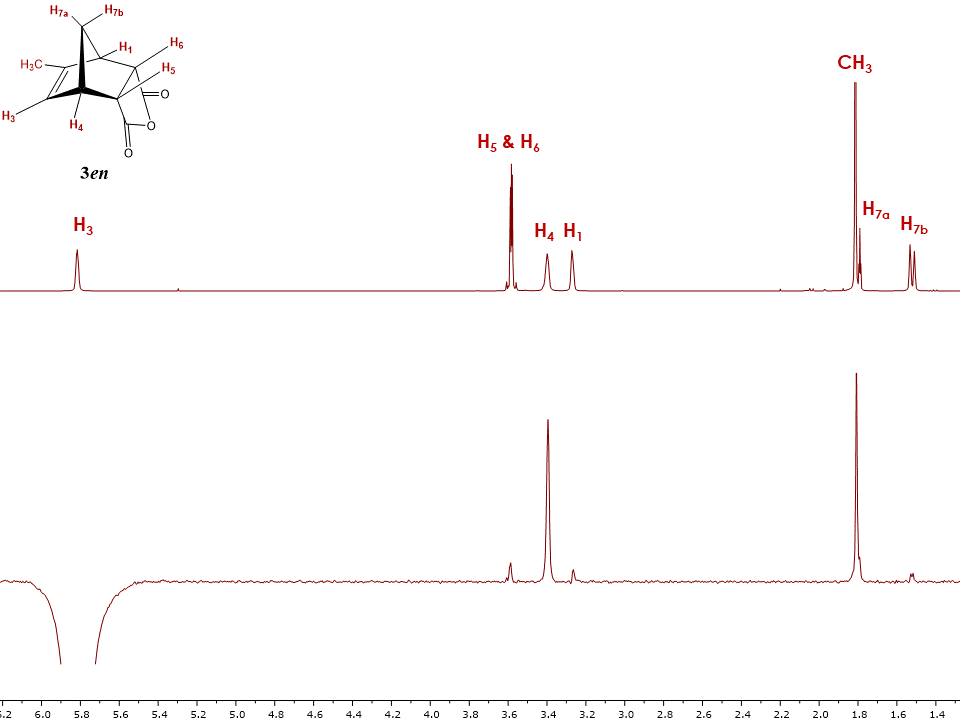
An environmentally friendly reagent, sodium hypochlorite, was used to oxidize the alcohol, and an IR spectrum was obtained in order to identify the starting compound and final product. Next, 15mL of sodium O-Vanillin Lab Report 840 Words 4 Pages As seen in table 1, the theoretical yield was. Tetraphenylnaphthalene Lab Report 779 Words 4 Pages Benzyne Formation and the Diels-Alder Reaction Preparation of 1,2,3,4 Tetraphenylnaphthalene Aubree Edwards Purpose: 1,2,3,4-tetraphenylnaphthalene is prepared by first producing benzyne via the unstable diazonium salt. The possible cause of the low percent yield maybe as the reaction was washed with hexane, the pipette may have removed some of the crystals. Thus, the maximum amount of moles of cis-Norbornene-5, 6-endo-dicarboxylic anhydride we can produce is: Now that the theoretical yield has been calculated, we can compare the experimental yield with the theoretical yield: Discussion The literature value for the melting point of cis-Norbornene-5, 6-endo-dicarboxylic anhydride is 165º C. The Diels- Alder reaction is one of the most important reactions in all of organic chemistry because of the applicability of it.

In this experiment, the melting point and of cis-Norbornene-5,6-endo-Dicarboxylic anhydride is determine. Causes serious eye damage. Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry. Throughout the experiment, the mixture changed color from green, orange, to yellowish lime, and eventually clear. Hence, the crystal lattice structure of the product is largely intact, requiring an even amount of thermal energy to melt the sample. Initially, the color of the reaction turned into a dark green color and over time became a lighter shade with a minimal solid left.