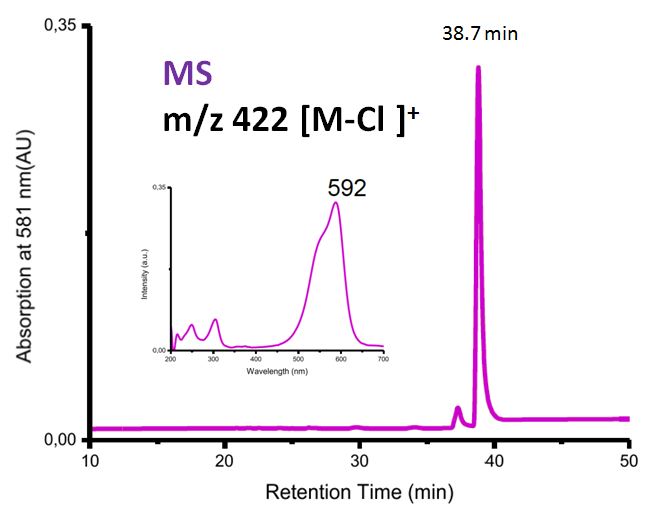
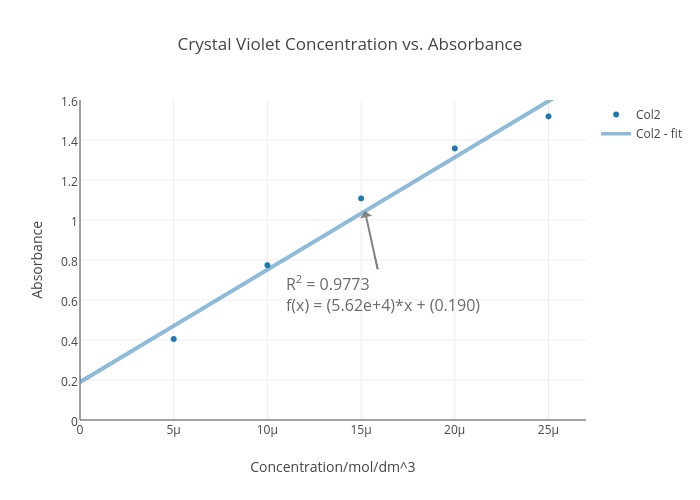
Crystal violet, also known as gentian violet or methyl violet, is a widely used dye in various scientific applications. One of the most common uses of crystal violet is to measure its absorbance, which refers to the amount of light absorbed by a substance. Absorbance is a crucial parameter in many analytical techniques, including spectrophotometry, which is the measurement of how much light is absorbed by a substance at different wavelengths.
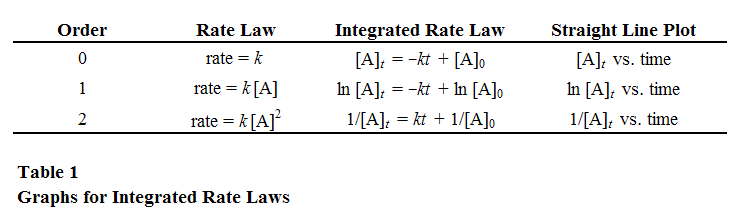
In spectrophotometry, the absorbance of a substance is usually measured by passing light through a solution containing the substance and measuring the amount of light that is absorbed. The absorbance is then used to calculate the concentration of the substance in the solution. This is done using the Beer-Lambert law, which states that the absorbance of a substance is directly proportional to its concentration.
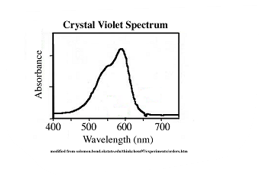
Crystal violet has a strong absorbance in the visible light spectrum, with a maximum absorbance at around 620 nm. This makes it an excellent choice for spectrophotometric analyses, as it allows for the precise measurement of its concentration in a solution.
One of the main advantages of using crystal violet for absorbance measurements is that it is a highly stable dye. It is resistant to fading and does not decompose easily, making it suitable for long-term storage. Additionally, crystal violet is inexpensive and widely available, making it a popular choice for many laboratories.
In addition to its use in spectrophotometry, crystal violet is also used in other applications where absorbance measurements are important. For example, it is often used as a stain in microscopy to highlight specific structures or cells. It is also used as a diagnostic tool in medicine, as it can be used to identify the presence of certain bacteria or fungi.
Overall, crystal violet is a versatile and widely used dye with many important applications in science and industry. Its strong absorbance in the visible light spectrum and stability make it an excellent choice for spectrophotometric analyses and other applications that require the measurement of absorbance.