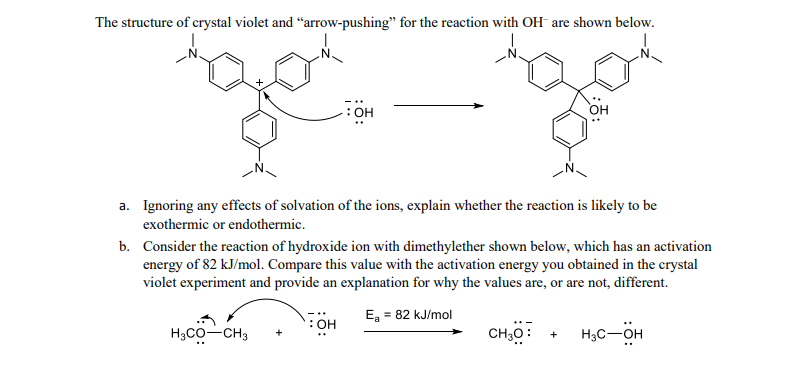
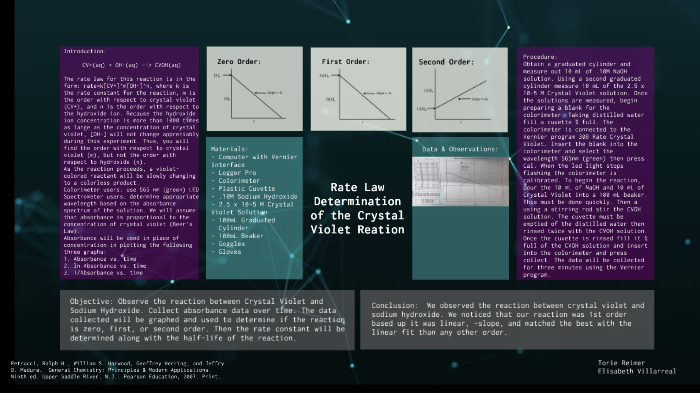
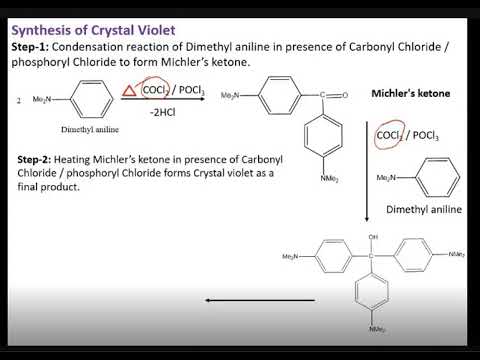
Crystal violet is a purple dye that is often used as a staining agent in microscopy, histology, and bacteriology. It is also known as gentian violet or methyl violet.
One of the most common uses of crystal violet is in the Gram staining procedure, which is used to differentiate between different types of bacteria. In this procedure, a bacterial sample is first fixed to a microscope slide, then treated with crystal violet. The crystal violet is absorbed by the bacteria, and then the slide is washed with a decolorizing solution. This removes the crystal violet from most bacteria, but some types of bacteria, called Gram-positive bacteria, retain the crystal violet because of their thick cell walls. These bacteria appear purple under the microscope, while Gram-negative bacteria, which have thinner cell walls and do not retain the crystal violet, appear pink or red after being treated with a counterstain called safranin.
The Gram staining procedure is widely used because it is simple, inexpensive, and reliable, and it can be used to identify many different types of bacteria. It is especially useful for identifying bacteria that are responsible for infections, such as streptococci, staphylococci, and pneumococci.
In addition to its use in microscopy, crystal violet is also used as a topical antifungal agent and a treatment for thrush (a fungal infection of the mouth and throat). It is also used as a dye in the manufacture of inks, paints, and other products.
Despite its many uses, crystal violet has some potential drawbacks. It can be toxic if ingested, and it may cause skin irritation or allergic reactions in some individuals. It is also classified as a potential carcinogen, although the evidence for this is limited and conflicting.
Overall, crystal violet is a widely used and versatile dye that has many important applications in science and medicine. Its ability to differentiate between different types of bacteria has made it an invaluable tool in the identification and treatment of bacterial infections, and its other uses as a dye and antifungal agent have made it a valuable resource in a variety of industries.