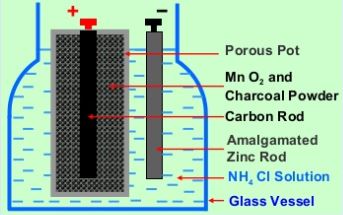
The Daniell cell is an electrochemical cell that was developed in the early 19th century by English chemist John Frederic Daniell. It was one of the first electrochemical cells to use a galvanic element, which is a substance that can spontaneously generate an electrical current through a chemical reaction. The Daniell cell is also known as a "wet cell" because it uses liquid electrolytes, as opposed to a dry cell, which uses a solid electrolyte.
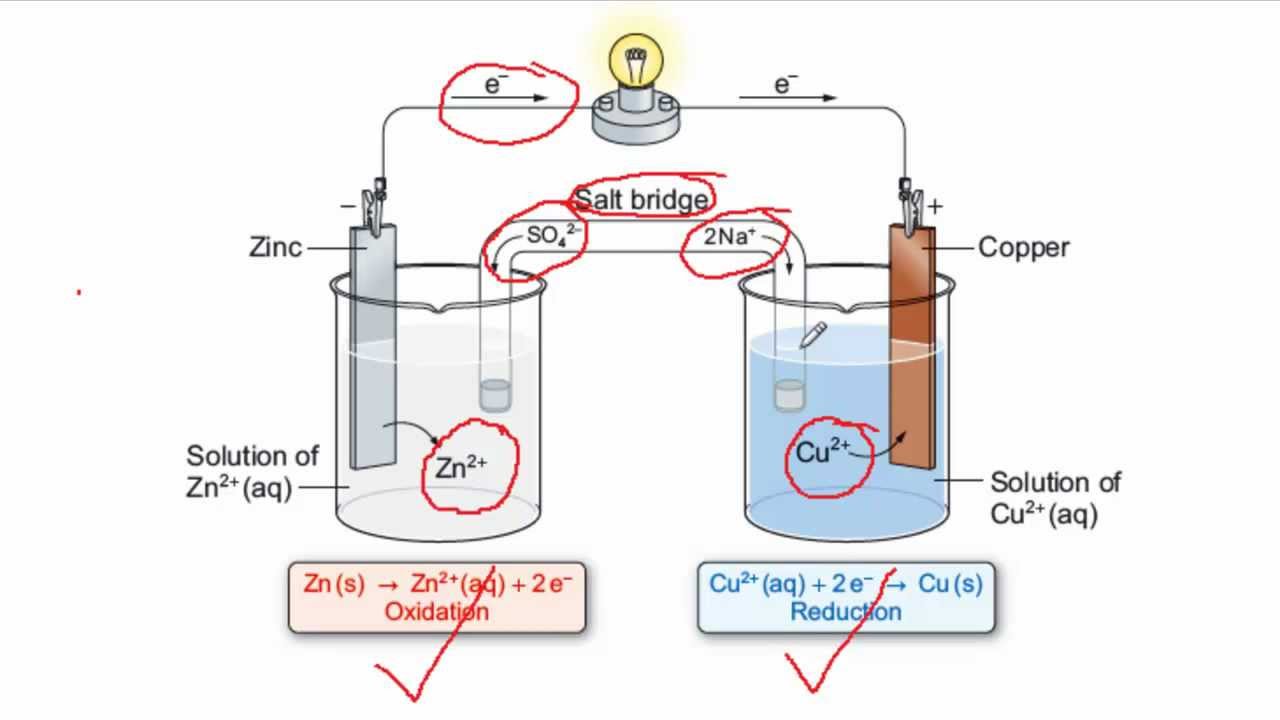
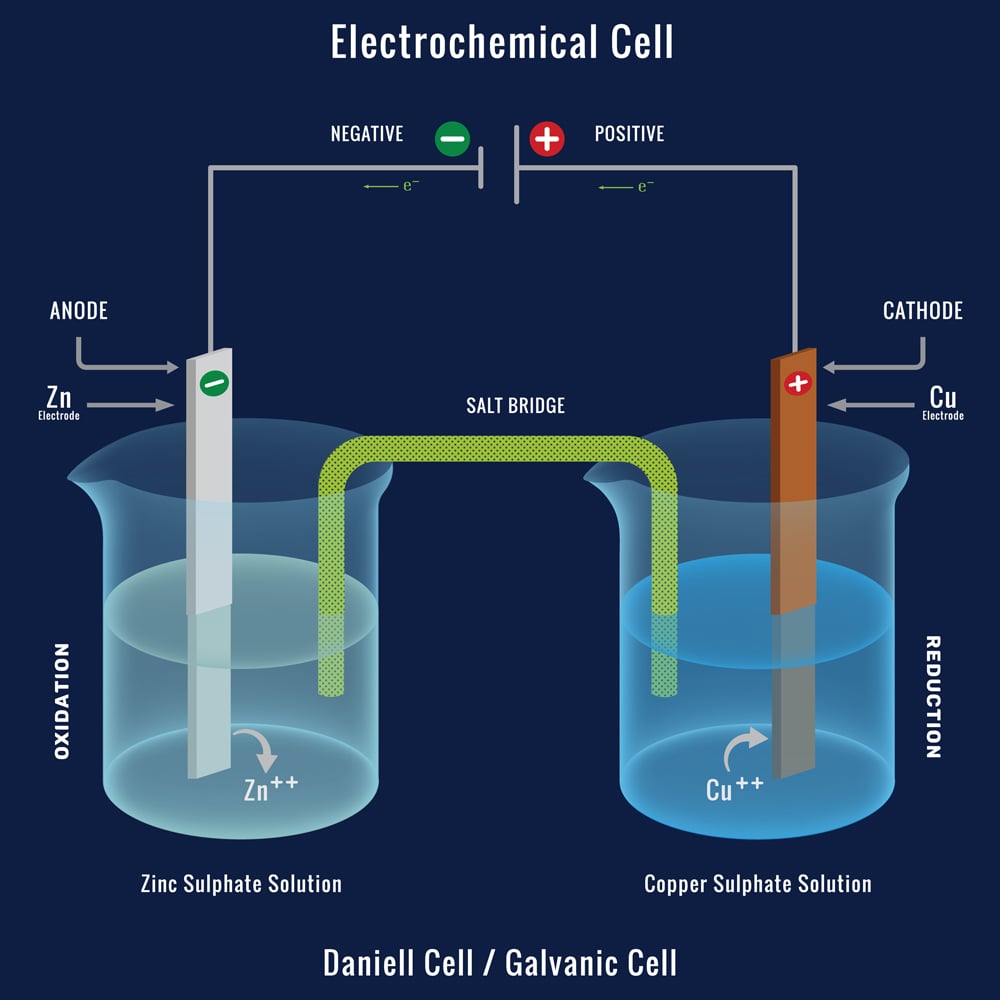
The Daniell cell consists of two half-cells, each containing a different metal electrode and an electrolyte solution. The two half-cells are connected by a salt bridge, which allows the flow of ions between the two half-cells. The half-cell that contains the zinc electrode is called the anode, and the half-cell that contains the copper electrode is called the cathode.
When the Daniell cell is connected to an external circuit, a chemical reaction occurs in the half-cells. In the anode half-cell, the zinc electrode reacts with the electrolyte solution, causing it to release electrons. These electrons flow through the external circuit to the cathode half-cell, where they are used to reduce copper ions in the electrolyte solution to copper metal. The copper metal then deposits on the copper electrode, creating a copper coating.
The overall chemical reaction that occurs in the Daniell cell can be represented by the following equation:
Zn + CuSO4 → ZnSO4 + Cu
In this equation, Zn represents the zinc electrode, CuSO4 represents the copper sulfate electrolyte solution, ZnSO4 represents the zinc sulfate byproduct, and Cu represents the copper metal that is deposited on the copper electrode.
The Daniell cell is a simple and reliable source of electricity, and it has a number of practical applications. It can be used as a power source for small electronic devices, such as calculators and flashlights, and it can also be used in scientific experiments to demonstrate the principles of electrochemistry.
In conclusion, the Daniell cell is an important historical development in the field of electrochemistry. It is a simple and reliable source of electricity that is still used today in a variety of practical applications.