Ethyl alcohol, also known as ethanol or grain alcohol, is a clear, colorless liquid with a characteristic fruity or sweet odor and taste. It is a commonly used solvent, flavorant, and psychoactive drug, and it is the active ingredient in alcoholic beverages. Ethyl alcohol has a number of physical and chemical properties that are of interest to researchers, including its density.
The density of a substance is a measure of its mass per unit volume. It is typically expressed in grams per milliliter (g/mL) or kilograms per liter (kg/L). The density of a substance is influenced by its molecular weight, the size and shape of its molecules, and the strength of the forces holding its molecules together.
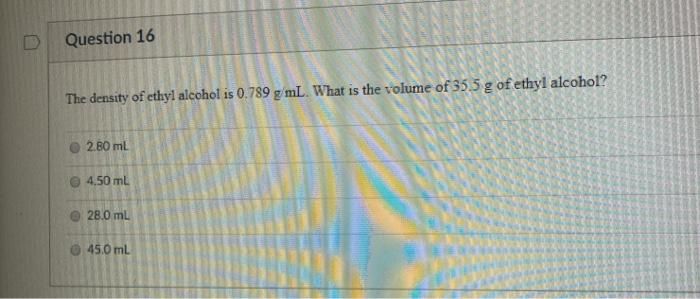
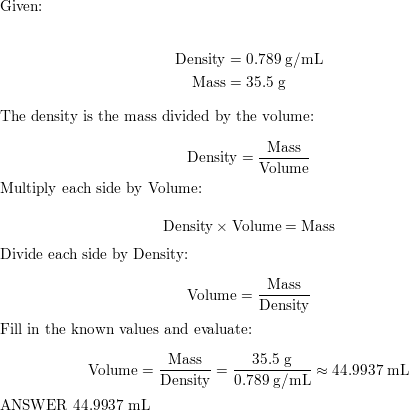
The density of ethyl alcohol is 0.789 g/mL at 20 degrees Celsius (68 degrees Fahrenheit). This value is relatively low, indicating that ethyl alcohol is less dense than water, which has a density of 1 g/mL at the same temperature. The lower density of ethyl alcohol is due in part to the fact that its molecules are smaller and less massive than those of water.
The density of ethyl alcohol also changes with temperature. Like many other liquids, it expands when heated and contracts when cooled. As a result, the density of ethyl alcohol decreases as its temperature increases. At 25 degrees Celsius (77 degrees Fahrenheit), for example, the density of ethyl alcohol is 0.785 g/mL, while at 30 degrees Celsius (86 degrees Fahrenheit) it is 0.780 g/mL.
In addition to its density, ethyl alcohol has a number of other properties that make it useful in a variety of applications. It is a good solvent for many organic compounds, and it is widely used as a solvent in the production of personal care and cleaning products. It is also a common component of food and beverages, where it is used as a flavorant and a preservative.
In conclusion, the density of ethyl alcohol is an important physical property that is influenced by its molecular structure, temperature, and other factors. It is a useful measure for researchers and industry professionals, as it can help them understand the behavior of this versatile and widely used chemical compound.