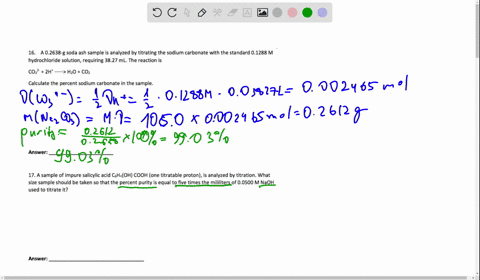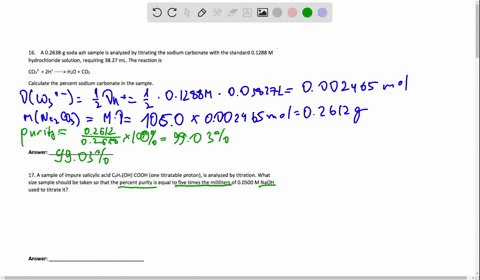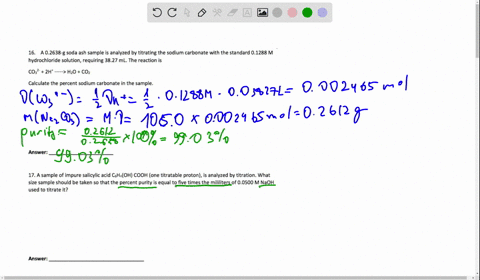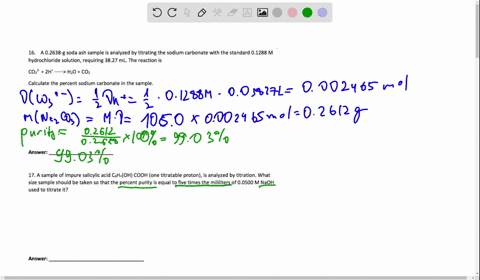
The shroud of silence refers to a phenomenon in which individuals or groups remain silent or avoid speaking out about a particular issue or topic, often due to fear of backlash or consequences. This silence can occur in a variety of contexts, including within families, communities, or even entire societies. It can also be motivated by a desire to protect oneself or others, maintain social norms, or avoid conflict.
One example of the shroud of silence can be seen in the way that victims of abuse often remain silent about their experiences. This can be due to fear of retribution from their abuser, feelings of shame or guilt, or a belief that no one will believe them or take their experiences seriously. The shroud of silence can also be seen in cases of discrimination, where individuals may be hesitant to speak out about their experiences for fear of being ostracized or facing further discrimination.
Another example of the shroud of silence can be seen in the way that certain topics or issues are avoided or discouraged from being discussed in certain contexts. For instance, there may be a societal taboo around discussing mental health, leading to a shroud of silence surrounding the topic. Similarly, certain political or social issues may be considered taboo or controversial, leading to a shroud of silence around them.
The shroud of silence can have significant negative consequences, including perpetuating harmful behaviors or beliefs, preventing individuals from seeking help or support, and hindering progress or change on important issues. Breaking the shroud of silence can be difficult and may require courage, but it can also be incredibly powerful and can lead to greater understanding, support, and ultimately, positive change.
In conclusion, the shroud of silence is a pervasive and harmful phenomenon that can have serious consequences for individuals and society as a whole. Breaking the shroud of silence and fostering an environment where open and honest communication is encouraged can be an important step towards creating a more just and equitable society.