Enzymes are proteins that catalyze chemical reactions in living organisms. One example of an enzyme is trypsin, which is found in the pancreas and helps to digest proteins in the small intestine.
In this experiment, we will be using trypsin to digest proteins in milk. Milk contains several different types of proteins, including casein, lactoglobulin, and lactalbumin. When milk is ingested, the trypsin in the small intestine breaks down these proteins into smaller peptides and amino acids, which can then be absorbed into the bloodstream.
To conduct the experiment, we will first need to obtain some trypsin from a laboratory supply company or prepare it ourselves using a protocol from a chemistry textbook. Next, we will mix the trypsin with milk in a test tube and incubate the mixture at 37°C for a period of time, typically several hours to a day.
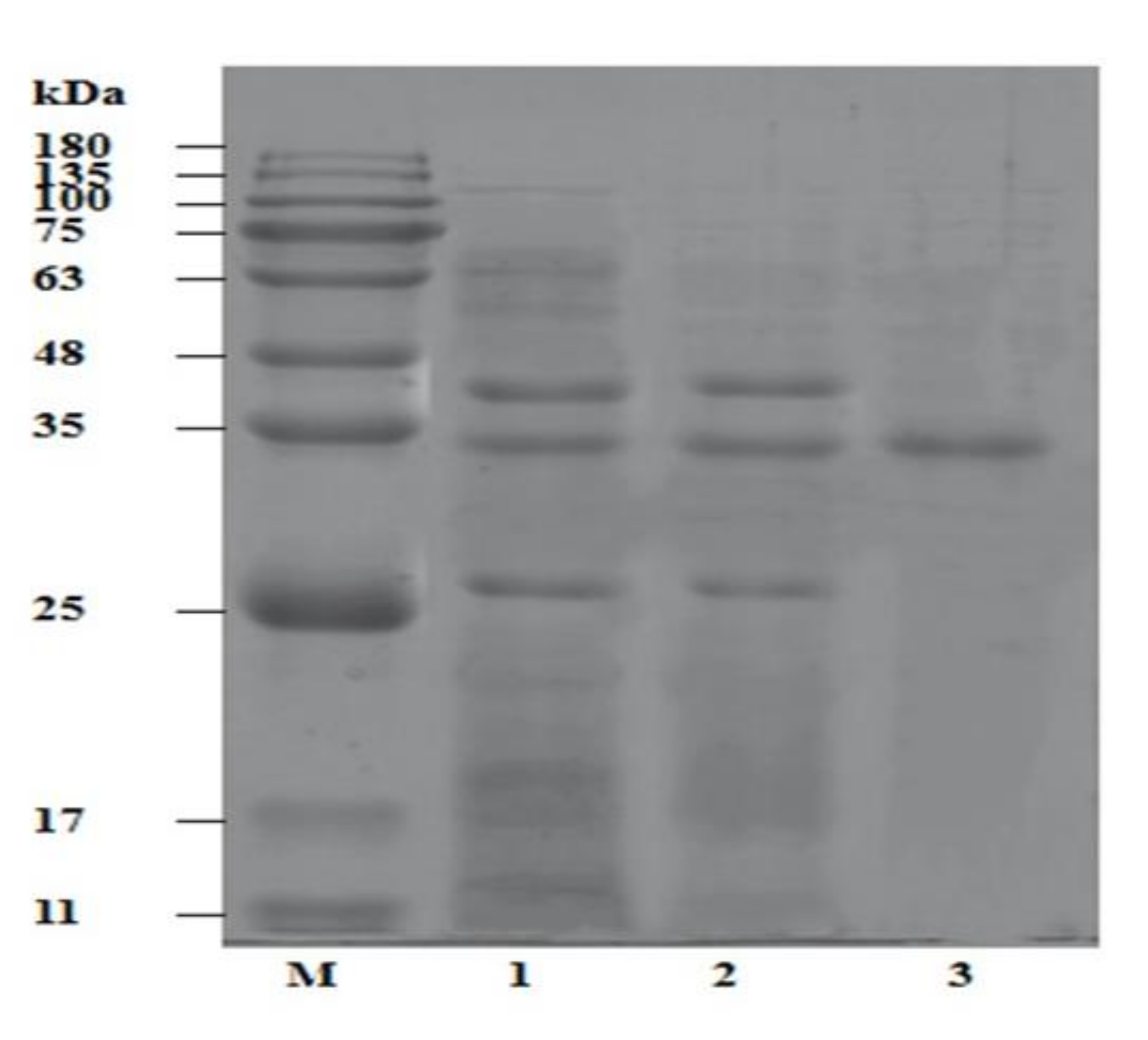
After the incubation period, we can observe the effects of the trypsin on the milk proteins by using techniques such as gel electrophoresis or spectrophotometry. Gel electrophoresis involves separating proteins based on their size and charge, while spectrophotometry measures the absorbance of light at different wavelengths, which can be used to determine the concentration of proteins in a solution.
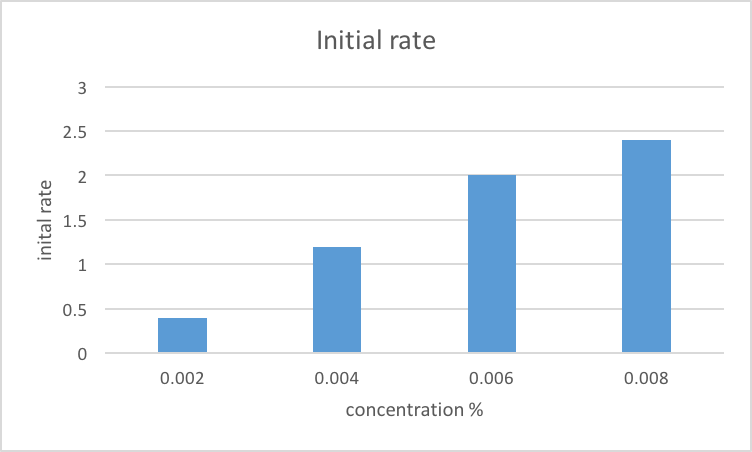
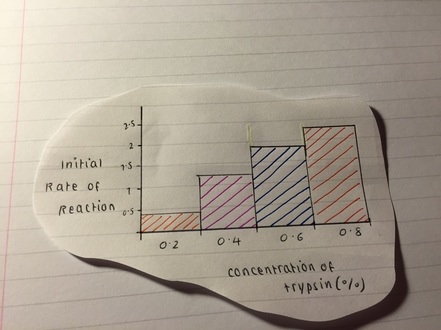
Using these techniques, we can observe how the trypsin has broken down the milk proteins and compare the results to a control group where no trypsin was added. We can also vary the concentration of trypsin and the incubation time to see how these factors affect the rate and extent of protein digestion.
Overall, the enzyme trypsin milk experiment is a useful way to study the role of enzymes in protein digestion and the factors that can affect enzyme activity. By understanding these processes, we can gain insight into how the body digests and utilizes proteins, which is important for maintaining good health and preventing diseases such as malnutrition and malabsorption.
.png)