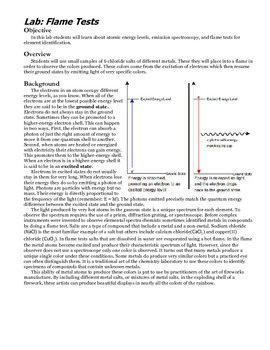
A flame test is a common laboratory technique used to identify the presence of certain elements in a sample by observing the colors of a flame when the sample is burned. The flame test can be used to identify a wide variety of elements, including metals and metalloids, and is often used in chemistry labs to identify unknown substances.
To perform a flame test, a small amount of the sample is placed on the end of a thin wire or platinum loop and held in the flame of a Bunsen burner. As the sample is burned, it will emit certain wavelengths of light that correspond to the energy levels of its atoms. These wavelengths of light are unique for each element and can be used to identify the element present in the sample.
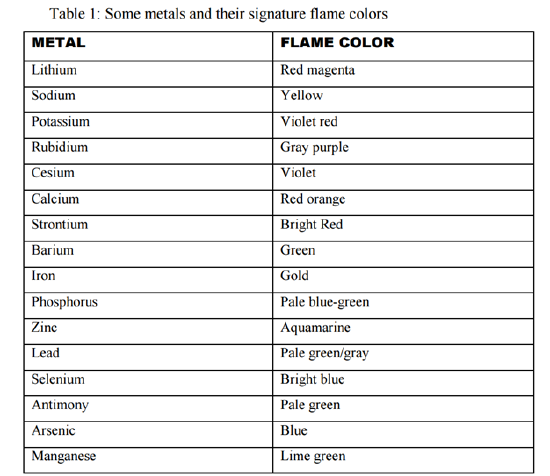
The colors produced by a flame test can range from deep red and orange to blue and green, depending on the element being tested. For example, sodium will produce a bright yellow flame, while copper will produce a green flame.
In order to accurately interpret the results of a flame test, it is important to use a standardized color chart that lists the expected flame colors for each element. It is also important to ensure that the sample being tested is pure and free of contaminants, as these can affect the color of the flame.
To write a lab report on a flame test, it is important to follow a standard scientific format. This typically includes an introduction, materials and methods, results, and discussion sections.
In the introduction, provide background information on the flame test and its purpose. This could include a brief overview of the history of the technique, as well as its uses in identifying elements.
In the materials and methods section, describe the steps taken to perform the flame test, including any equipment or chemicals used. Be sure to include details on how the samples were prepared and how the flame colors were observed and recorded.
In the results section, present the data from the flame test, including any observations made and any patterns that emerged. This could include tables or graphs to help illustrate the results.
In the discussion section, interpret the results of the flame test and draw conclusions about the identity of the elements present in the sample. Be sure to consider any potential sources of error or uncertainty in the results, and discuss any implications of the findings.
Overall, a flame test is a useful and widely used technique for identifying the presence of certain elements in a sample. By following a standard scientific format and carefully documenting the results, it is possible to accurately report on the findings of a flame test lab experiment.