In "Two Kinds," Amy Tan explores the complex and strained relationship between a Chinese immigrant mother and her American-born daughter. The daughter, Jing-mei, struggles to reconcile her mother's expectations for her to become a prodigy with her own desires to forge her own path in life. The mother, Suyuan, is driven by a fierce determination to give her daughter every opportunity for success, fueled by the belief that American culture is superior to Chinese culture and that being successful in America will bring her daughter respect and acceptance.
Through the use of flashbacks, Tan delves into the history of Suyuan's past in China and how she lost everything in the war, including her twin daughters. Suyuan's experiences have shaped her belief that Jing-mei must succeed at all costs, and she pushes her daughter to be a prodigy in piano, math, and other subjects. Jing-mei, on the other hand, resists her mother's expectations and ultimately rebels by refusing to continue with the piano lessons.
The conflict between Suyuan and Jing-mei ultimately comes to a head when Jing-mei discovers that she has a half-sister in China, a revelation that forces her to confront the fact that her mother has been keeping secrets from her and that her own identity is more complex than she had previously thought. Through this revelation, Jing-mei begins to understand her mother's motivations and the sacrifices that Suyuan has made for her daughter's future.
The thesis statement for "Two Kinds" could be: In "Two Kinds," Amy Tan uses the strained relationship between a Chinese immigrant mother and her American-born daughter to explore the complexities of identity, expectations, and cultural differences.
Copper(II) sulfate
CuSO4 is copper II sulfate that has the copper metal in a +2 oxidation state. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, Roman vitriol. The crucible was then weighed and the weight was recorded. Murray and others, Edinburgh. Support the crucible securely in the pipe-clay triangle on the tripod over the Bunsen burner.
Copper Sulfate Formula

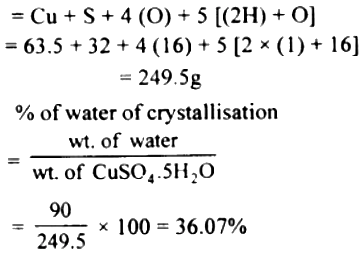
Record all weighings accurate to the nearest 0. Subsequently, a paper clip was placed in the crucible. Complex ions are compounds having a central atom surrounded by other molecules called ligands, and these ligands can form dative covalent bonds to the central particle Lister and Renshaw 2000. Thus, the product is called copper sulfate pentahydrate. It can be deduced that when over heating, the black CuO s and the pungent smell SO3 g would be observed. In industry copper sulfate has multiple applications. So the difference is anhydrous copper II sulfate does not have water of crystallization and is a white powder.
Finding_the_formula_of_hydrated_copper

What are the two differences between hydrated and anhydrous copper sulphate? In part 2, the steps making complex ions in solution can be shown below Lane, 2009. Controlling the flame intensity of burner, putting an asbestos net under the crucible or granulating the crystal can reduce the possibility of decomposition and ensure the crystal is dehydrated completely. This information is used to find x in the formula CuSO 4. Copper II oxide CuO and sulfur trioxide SO3 will be produced when heating the crystal at around 600? Anhydrous copper sulphate is an inorganic compound having only copper sulphate molecules in its chemical structure. The colour changing grey-white to blue when adding water into anhydrous copper sulphate can explain why the crucible needs to cool down inside the desiccator. Before placing the crucible on the stand and heating, the burner was lit and placed under the stand. Thermal decomposition of ionic solids.
Hydrated Copper Sulfate Formula

The five in front of the formula for water tells us there are 5 water molecules per formula unit of CuSO4 or 5 moles of water per mole of CuSO4. It forms CuSO 4· nH 2O, where n can range from 1 to 7. Copper II sulfate pentahydrate is the pentahydrate of copper 2+ sulfate. In part 2, the steps making complex ions in solution can be shown below Lane, 2009. Water molecules can make the copper complex ion blue. The crucible may not be completely dry, and extra water evaporated will give a higher value. The method of this experiment was divided into two parts.






