Anhydrous sodium carbonate, also known as soda ash, is a white, odorless, crystalline solid with a formula of Na2CO3. It is a highly alkaline compound, with a pH of around 11.2 in aqueous solution.
Sodium carbonate is a versatile compound with a wide range of applications, including use as a water softener, detergent booster, and pH adjuster. It is also used in the manufacturing of glass, soap, and paper, as well as in the refining of petroleum products.
The chemical formula for anhydrous sodium carbonate reflects its molecular structure, which consists of two sodium atoms bonded to a single carbon atom, which is then bonded to three oxygen atoms. The compound is an ionic compound, meaning that it is composed of positively charged ions (sodium) and negatively charged ions (carbonate).
Sodium carbonate can be synthesized through a variety of methods, including the Solvay process and the ammonia-soda process. In the Solvay process, salt, limestone, and ammonia are combined to produce sodium bicarbonate, which is then heated to produce anhydrous sodium carbonate. The ammonia-soda process involves the electrolysis of a sodium chloride solution to produce chlorine and sodium hydroxide, which are then used to produce sodium carbonate.
Anhydrous sodium carbonate is a stable compound under normal conditions, but it will decompose when heated to high temperatures, releasing carbon dioxide gas. It is also soluble in water, forming a strong alkaline solution.
Overall, anhydrous sodium carbonate is an important chemical compound with a wide range of uses in industry and household products. Its formula, Na2CO3, reflects its molecular structure and the arrangement of its atoms, which contribute to its chemical properties and reactivity.
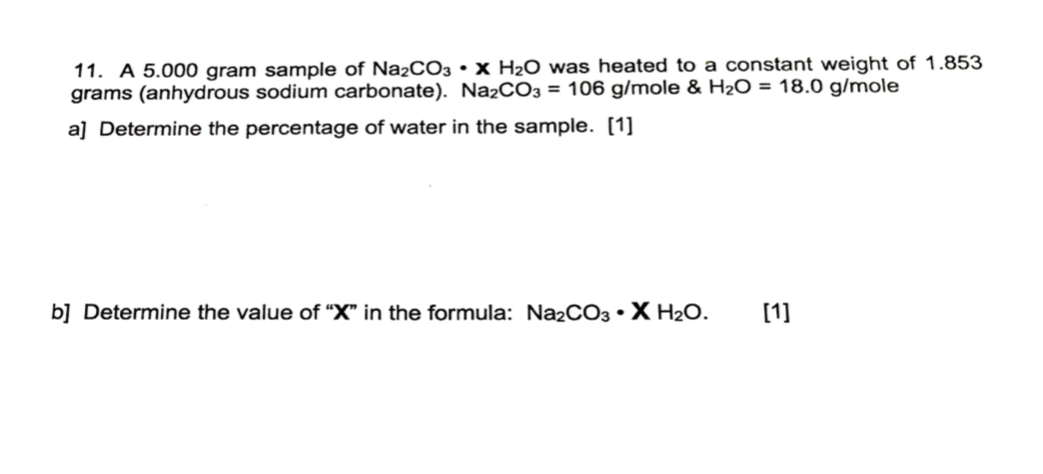
[Solved] What is the formula mass of anhydrous sodium carbonate? (Giv
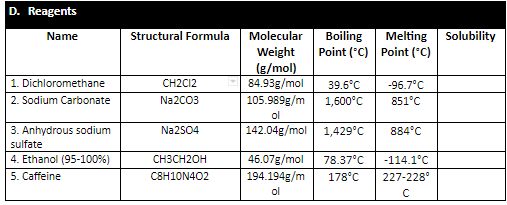
IDENTIFICATION Product Name: Sodium Carbonate, Anhydrous Product Number: All applicable American Elements product codes, e. Direct skin or eye contact, or inhalation of powder or crystals can produce irritation, rash and sometimes burns. Solutions are packaged in polypropylene, plastic or glass jars up to palletized 440 gallon liquid totes, and 36,000 lb. Sodium Carbonate, on the other hand, has a low toxicity risk for the most part. The above chemical reaction is unbalanced. Pack Sizes: 250g, 1Kg, 2.
In anhydrous sodium carbonate? Explained by FAQ Blog

FIRST AID MEASURES Description of first aid measures If inhaled: Supply patient with fresh air. Sodium carbonate, or soda ash, Na 2CO 3, is widely distributed in nature, occurring as constituents of mineral waters and as the solid minerals natron, trona, and thermonatrite. A high pH harms aquatic organisms. Keep container tightly sealed. In anhydrous sodium carbonate? The conditions or methods of handling, storage, use and disposal of the product s described are beyond the control of Flinn Scientific, Inc. Sodium carbonate soda ash plays an important role as a water-soluble builder and co-builder in phosphate-containing and non-phosphate detergents.
Sodium carbonate

Why a base like sodium carbonate is used in the extraction process of caffeine? The person should not be given anything to eat or drink while they're unconscious. Sodium carbonate is also used as a relatively strong base in various settings. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of 22. Is sodium carbonate found in nature? This Safety Data Sheet SDS is for guidance and is based upon information and tests believed to be reliable. The data should not be confused with local, state, federal or insurance mandates, regulations, or requirements and CONSTITUTE NO WARRANTY.