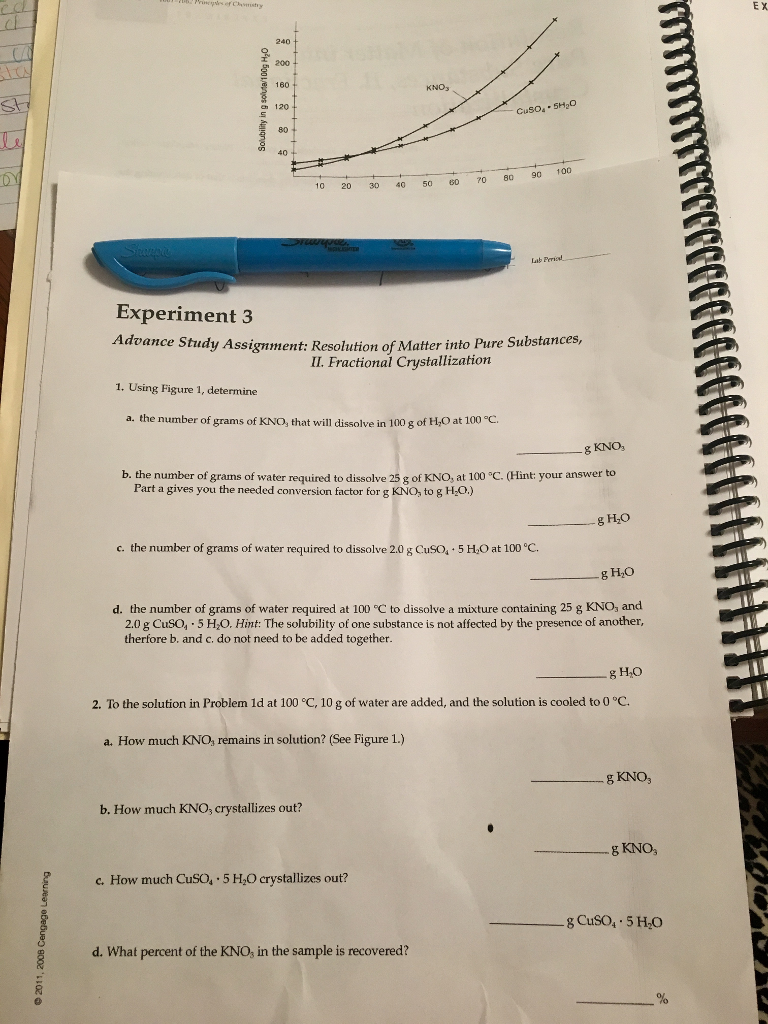
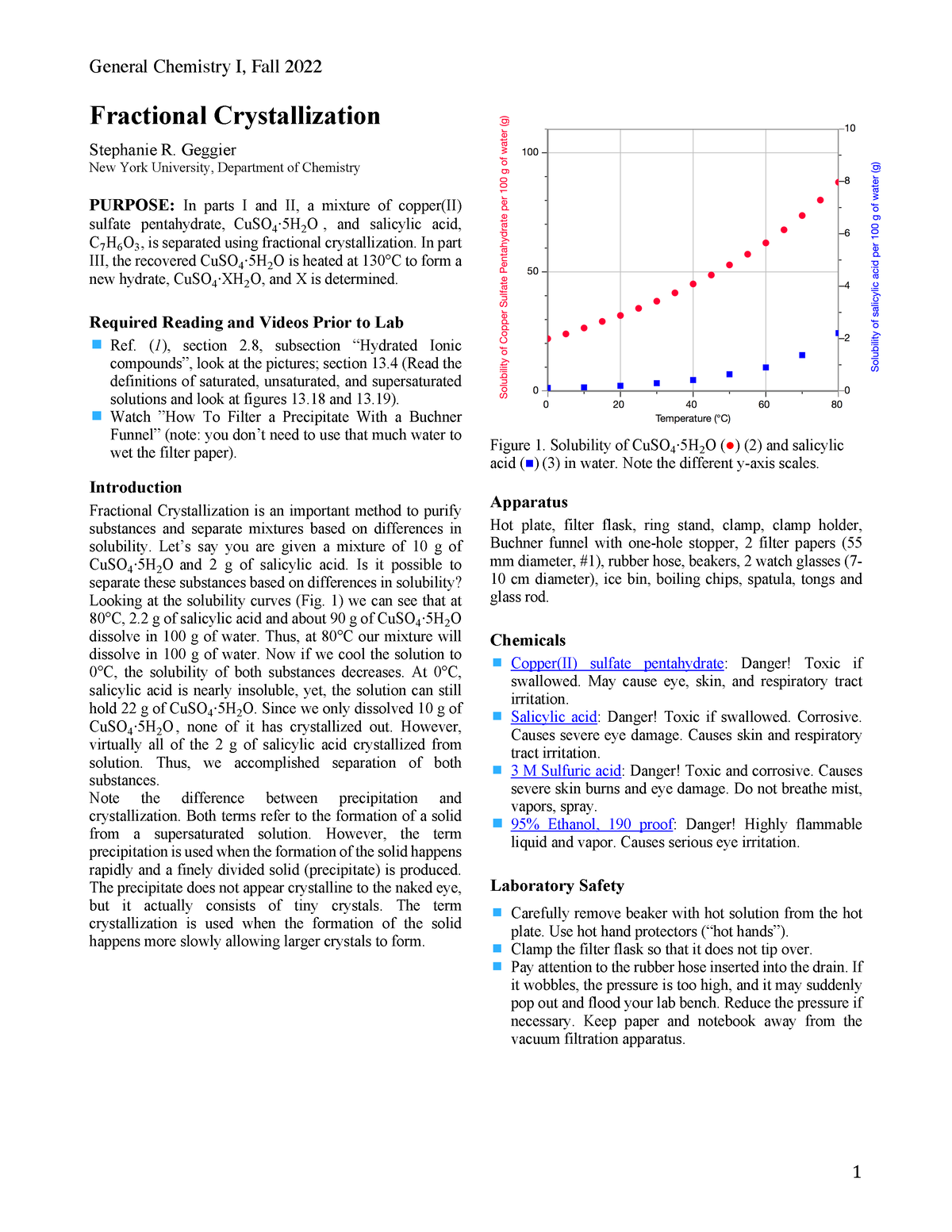
Fractional crystallization is a laboratory technique used to separate and purify a mixture of chemical compounds. It is based on the different solubilities of the components in a mixture at different temperatures or pH values. By carefully controlling the conditions in which a mixture is allowed to crystallize, it is possible to selectively precipitate out one or more of the components, leaving the others in solution.
In a fractional crystallization lab, a mixture of compounds is dissolved in a solvent and then allowed to cool or be exposed to a changing pH. As the solution cools or the pH changes, some of the compounds will begin to crystallize out of the solution. These crystals can then be separated from the remaining solution by filtration or centrifugation.
The key to successful fractional crystallization is the careful control of the conditions under which the mixture is allowed to crystallize. The temperature or pH must be changed gradually, in small increments, to allow the most soluble compound to crystallize out first. This process is repeated until all of the desired compounds have been separated.
There are several factors that can affect the success of a fractional crystallization experiment. The purity of the starting materials is important, as impurities can interfere with the crystallization process. The size and shape of the crystals can also be important, as larger or more irregularly shaped crystals may be more difficult to separate from the solution. Finally, the solubility of the compounds in the solvent used can also affect the outcome of the experiment.
Despite these challenges, fractional crystallization is an important tool in the purification of chemicals and has a wide range of applications in both industry and research. It is commonly used in the production of pharmaceuticals, dyes, and other chemical products, as well as in the isolation of natural products from plant and animal sources.
In conclusion, fractional crystallization is a powerful technique for separating and purifying mixtures of chemical compounds. By carefully controlling the temperature or pH of a solution, it is possible to selectively precipitate out one or more of the components, leaving the others in solution. This process has a wide range of applications in both industry and research, and is an important tool in the purification of chemicals.