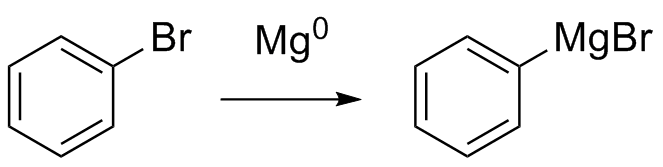
Grignard synthesis is a chemical reaction in which a Grignard reagent is used to synthesize a compound by reacting with an electrophile. A Grignard reagent is a compound containing a magnesium atom bonded to a carbon atom, which is bonded to a halogen atom (such as chlorine or fluorine) and a hydrocarbon group. The Grignard reagent is highly reactive and can be synthesized by reacting an organic halide with magnesium metal in diethyl ether or another suitable solvent.
The Grignard reaction is an important tool in organic chemistry for synthesizing a wide variety of compounds, including alcohols, amines, and ketones. It is often used in the production of pharmaceuticals and other organic compounds.
The reaction begins by adding the Grignard reagent to the solvent, which is typically diethyl ether. The Grignard reagent then reacts with the solvent to form a complex called a Grignard compound. This compound is highly reactive and can be used to synthesize a variety of compounds by reacting with electrophiles, which are compounds that are electron deficient and can accept a pair of electrons.
One of the most common electrophiles used in Grignard synthesis is carbon dioxide, which can be used to synthesize carboxylic acids. The Grignard reagent reacts with carbon dioxide to form a carboxylate salt, which can then be hydrolyzed to form a carboxylic acid.
Other electrophiles that can be used in Grignard synthesis include aldehydes, ketones, esters, and halogenated compounds. The Grignard reagent can also be used to synthesize amines by reacting with nitrogen-containing compounds such as amides or nitriles.
The Grignard reaction is a powerful tool for synthesizing a wide variety of compounds, and it has many applications in the pharmaceutical and chemical industries. However, it is important to handle Grignard reagents with caution due to their high reactivity and potential for explosion.
Grignard reaction

It can remove protons hydrogen atoms from alcohols, water, and carboxylic acids. If the level of the ether has decreased, add more. Just prior to your experiment carefully remove dry glassware from the oven, assemble and immediately cap it with a septum. The standard techniques for initiation of Grignard reactions is the addition of small amounts of 1,, methyl iodide or like compounds or the addition of preformed Grignard reagent to the solvents containing a small percentage of the total halide to be reacted. Assuming that you are starting with CH 3CH 2MgBr and using the general equation above you get always has the form: Since both R groups are hydrogen atoms, the final product will be: A primary alcohol is formed. In the first stage, the Grignard reagent adds across the carbon-oxygen double bond: Dilute acid is then added to this to hydrolyse it. As the acid is added the triphenylmethanol will separate from solution as a white precipitate.
7: The Grignard Reaction (Experiment)
One-Pot Access to 1-Azaspirocyclic Framework V. Grignard reagents include an R group, attached to a magnesium, attached to a halogen, often Bromide. The mixture is then brought to the boiling point and refluxed as long as hydrogen is evolved usually no longer than 5 minutes. Thus, there are two possible ways to utilize this invention; and both have their advantages: Both hydrides I and II are soluble in aromatic hydrocarbons and ethers. Szymoniak, Synthesis, 2002, 1115-1120. Grignard reactions are notoriously sensitive to water, and two approaches to controlling H 2O are shown in Figure 6. To increase the reaction time, a stirring rod was used to mash the magnesium in the presence of the mixture.
Grignard Reaction

Weigh magnesium powder 50 mg, 2 mmol and add it to your reaction vessel. The plate was developed using 90:10 hexane: ethyl acetate. The Grignard reaction, namely treatment of an organic halide with magnesium turnings in an ethereal solvent, is the main method for the preparation of organomagnesium compounds and has been thoroughly studied since the first report in 1900 Equation 15. They have a carbon-metal bond, which changes the polarity of a carbon atom from the partial positive charge in aryl and alkyl halides to the partial negative charge in Grignard reagents. The intermediate organosamarium displays considerable stability against β-elimination, suppressing the formation of the undesirable glucal side product. A reagent is a substance that is used to trigger a chemical reaction or to check the occurrence of a chemical reaction. These are: Both of these hydrides are viscous liquidsfrniscible with aromatic hydrocarbons.
US3758620A
Introduction Grignard reagents belong to a large class of organic compounds that are called organometallic reagents that has the formula R-M, with M being the metal. One of the R groups is hydrogen and the other CH 3. The Grignard reagents RMgX was prein example l-were charged into a 500cc three-necked 5 pared as described in Example 2. This can be combined, if necessary, with the addition of an activator 1,2-dibromoethane or iodine. The final product was purified and characterized using TLC and IR spectroscopy. Apparently the bis-fluoro impurity did not pose a problem with processing. In the first, you get an addition of the Grignard reagent to the carbon dioxide.