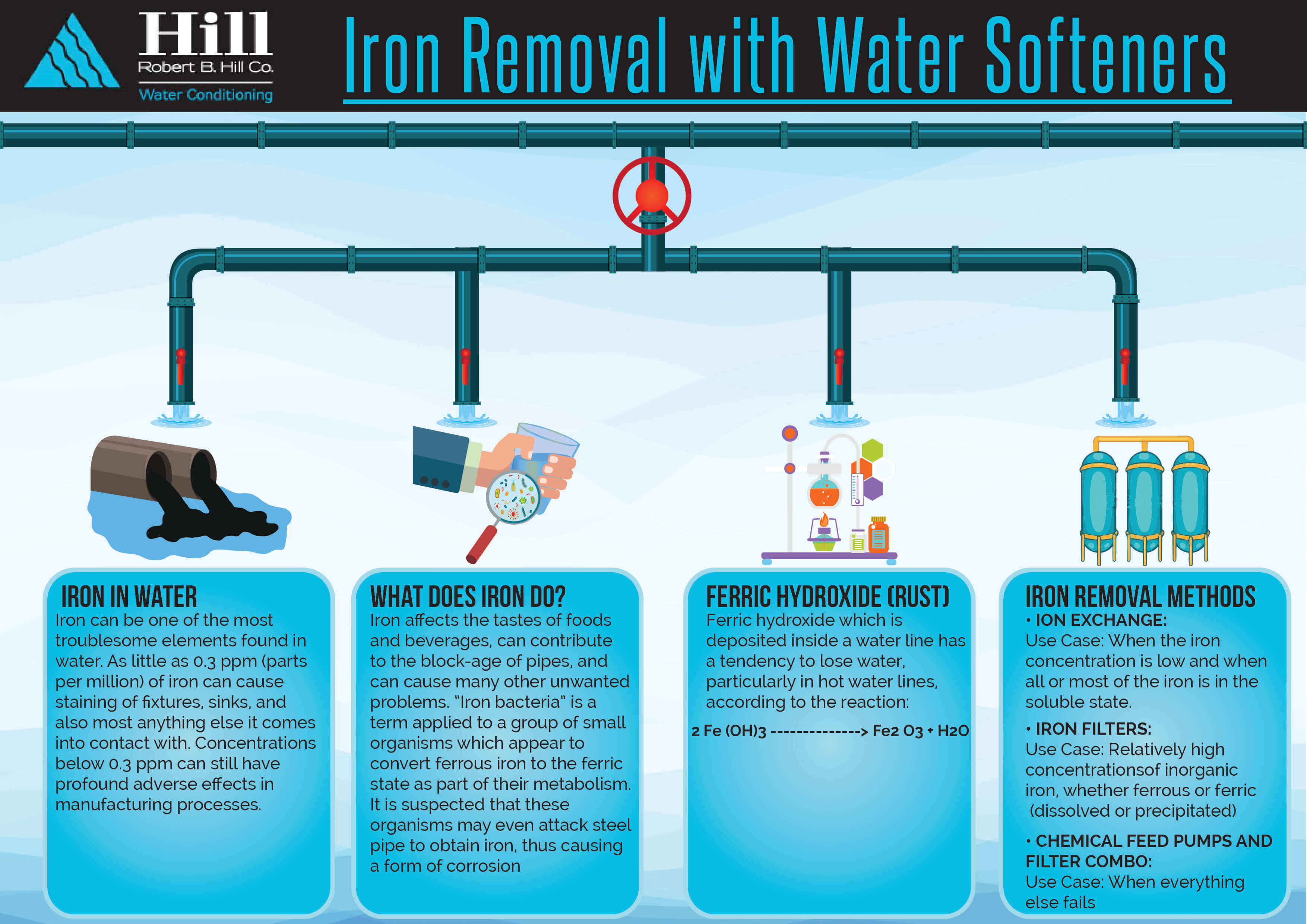
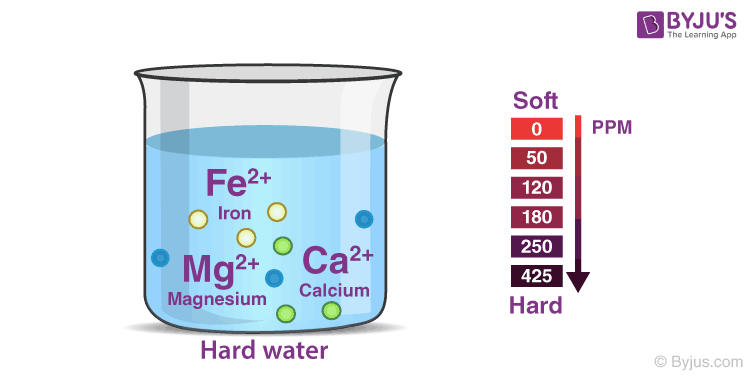
Hardness in water refers to the presence of dissolved minerals, specifically calcium and magnesium ions. These ions can cause a variety of problems, including making it difficult to lather soap and shampoo, leaving soap scum on dishes and clothing, and contributing to the formation of scale on pipes and appliances. Therefore, it is often necessary to remove hardness from water to improve its quality and reduce these negative effects.
There are several methods for removing hardness from water, each with its own advantages and limitations. These methods can be broadly classified into two categories: physical and chemical methods.
Physical methods for removing hardness involve mechanically separating the dissolved minerals from the water. One common method is distillation, which involves boiling the water and collecting the steam, which is then cooled and condensed back into a liquid form. The minerals are left behind in the original container, leaving the distilled water relatively free of hardness. However, this method is energy-intensive and can be costly, and it does not remove other contaminants from the water.
Another physical method is reverse osmosis, which involves forcing water through a semipermeable membrane that allows water molecules to pass through but traps the minerals. This method is relatively effective at removing hardness and other contaminants, but it requires a high pressure pump to force the water through the membrane, and the membranes need to be replaced periodically.
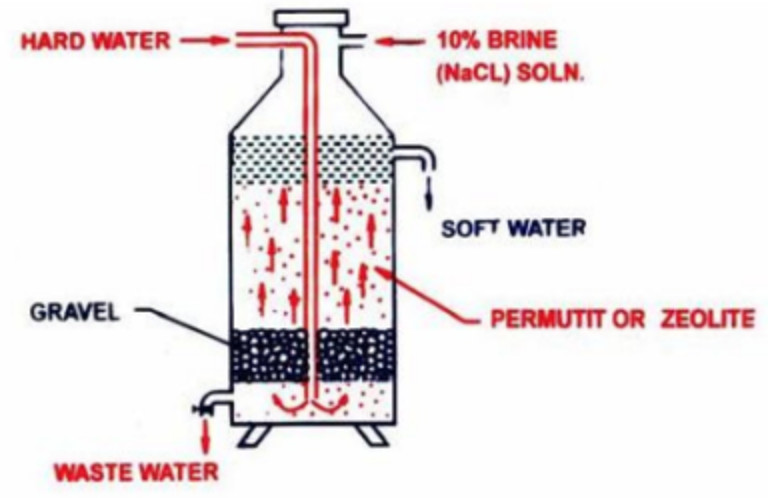
Chemical methods for removing hardness involve introducing chemicals into the water to either precipitate the minerals out of solution or to exchange them with other ions. One common chemical method is called ion exchange, which involves passing the water through a bed of resin beads that have been treated with a chemical that exchanges its ions with the calcium and magnesium ions in the water. The resin beads can be regenerated with a chemical solution when they become saturated with minerals, allowing the process to be repeated.
Another chemical method is called lime softening, which involves adding lime (calcium hydroxide) to the water to cause the calcium and magnesium ions to precipitate out as calcium carbonate and magnesium hydroxide. These precipitates can then be removed through sedimentation or filtration. Lime softening is relatively simple and effective, but it requires the addition of chemicals and can result in an increase in the pH of the water.
In conclusion, there are several methods for removing hardness from water, each with its own advantages and limitations. Physical methods, such as distillation and reverse osmosis, are relatively effective but can be costly and energy-intensive. Chemical methods, such as ion exchange and lime softening, are simpler and often more cost-effective, but they require the addition of chemicals and can have other impacts on the water quality. The most appropriate method will depend on the specific needs and resources of the user.