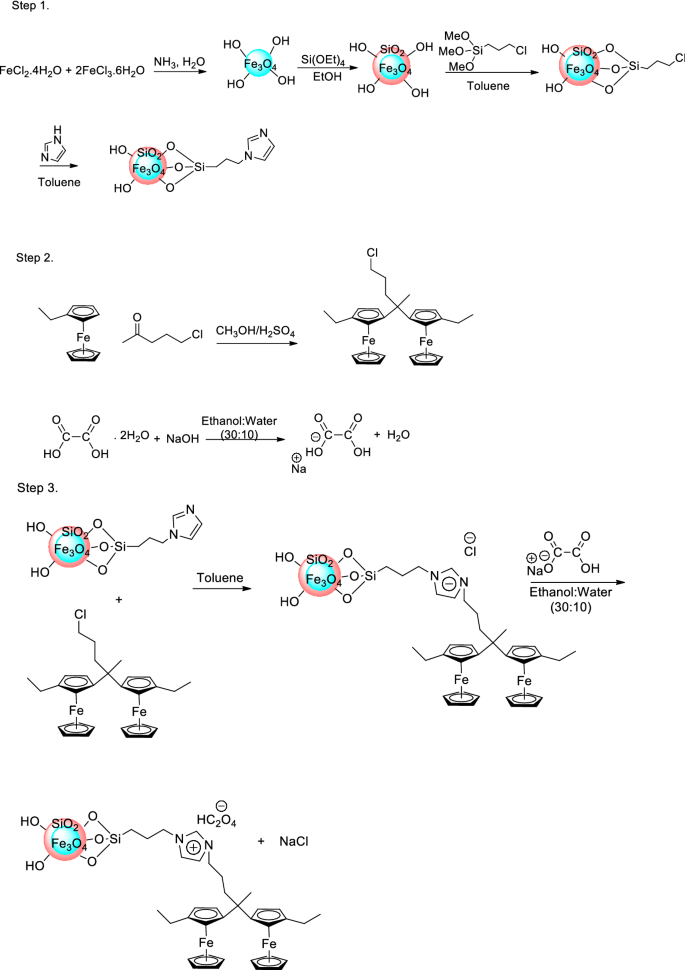
HC2O4 is the chemical formula for peracetic acid, a strong oxidizing agent that is commonly used as a disinfectant and sanitizer.
Peracetic acid is a clear, colorless liquid with a pungent, vinegar-like odor. It is a highly reactive compound that is easily decomposed by heat and light, and it is typically stored in brown or amber-colored bottles to protect it from exposure to sunlight.
Peracetic acid is used in a variety of applications, including the food industry, where it is used to sanitize food processing equipment and surfaces. It is also used in the healthcare industry to disinfect surfaces and medical instruments, and in the water treatment industry to kill bacteria and other microorganisms in drinking water.
Peracetic acid is a powerful disinfectant because it is able to penetrate the cell walls of microorganisms and disrupt their metabolism. It is effective against a wide range of bacteria, fungi, and viruses, including E. coli, Salmonella, and Listeria.
Despite its effectiveness as a disinfectant, peracetic acid can be hazardous to human health if inhaled or ingested. It is classified as a severe eye irritant and can cause respiratory irritation and burning in the throat and chest. It is important to handle peracetic acid with caution and to use it in a well-ventilated area to minimize the risk of exposure.
Overall, peracetic acid, or HC2O4, is a useful and effective disinfectant and sanitizer that is widely used in a variety of industries. However, it is important to handle it with care and to follow proper safety procedures when using it to minimize the risk of injury or illness.
Molar mass of H2C2O4*2H2O
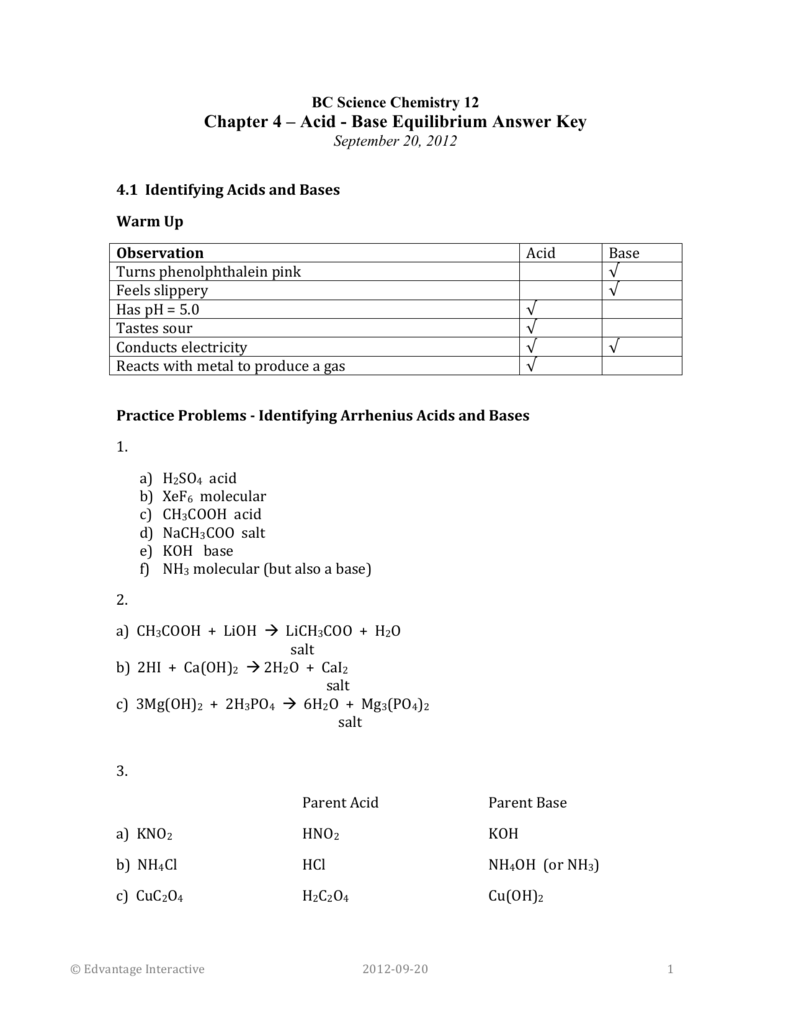
Ullmann's Encyclopedia of Industrial Chemistry. The Journal of Chemical Physics. Is calcium hydroxide acidic or basic? Series A, Containing Papers of a Mathematical and Physical Character. Retrieved 2 January 2019. Calcium hydroxide, also known as slaked lime with the chemical formula Ca OH 2 is a source of hydroxide ions when dissolved in aqueous solutions. H3AsO4 is a stronger acid than HAsO42- because it has more acidic H atoms. Is H2C2O4 a strong or weak electrolyte? BASE wikipedia In chemistry, bases are substances that, in aqueous solution, are slippery to the touch, taste astringent, change the color of indicators e.
Oxalate
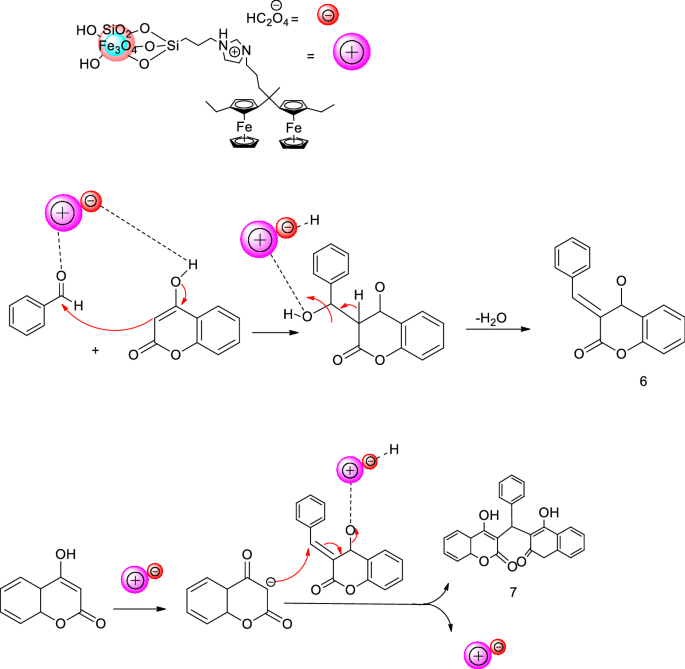
Proceedings of the Royal Society of London. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. NEUTRAL wikipedia In chemistry, neutralization or neutralisation see spelling differences , is a chemical reaction in which an acid and a base react quantitatively with each other. Hydrogenoxalate is Well characterized salts include NaHC 2O 4 , KHC 2O 4 , NH 3HC 2O 4 , rubidiumhydrogenoxalate RbHC 2O 4 CH 3 2 NH 2HC 2O 4. A compound with the molecular formula H2C2O4 has a C-C single bond, and no bonds between oxygen atoms.
Is H2C2O4 an acid or base or neutral ?
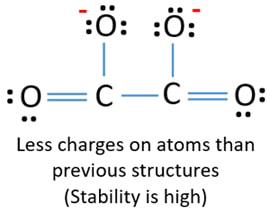
Question : Is H2C2O4 an acid or base or neutral? A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. Is H2C2O4 ionic or covalent? What type of bonding is H2C2O4? New England Journal of Medicine. Oxalic acid is a weak acid and will ionize in an aqueous solution. Retrieved 10 June 2021. Acta Crystallographica Section B. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl, Al. Urolithiasis, A Medical and Surgical Reference.
Hydrogenoxalate

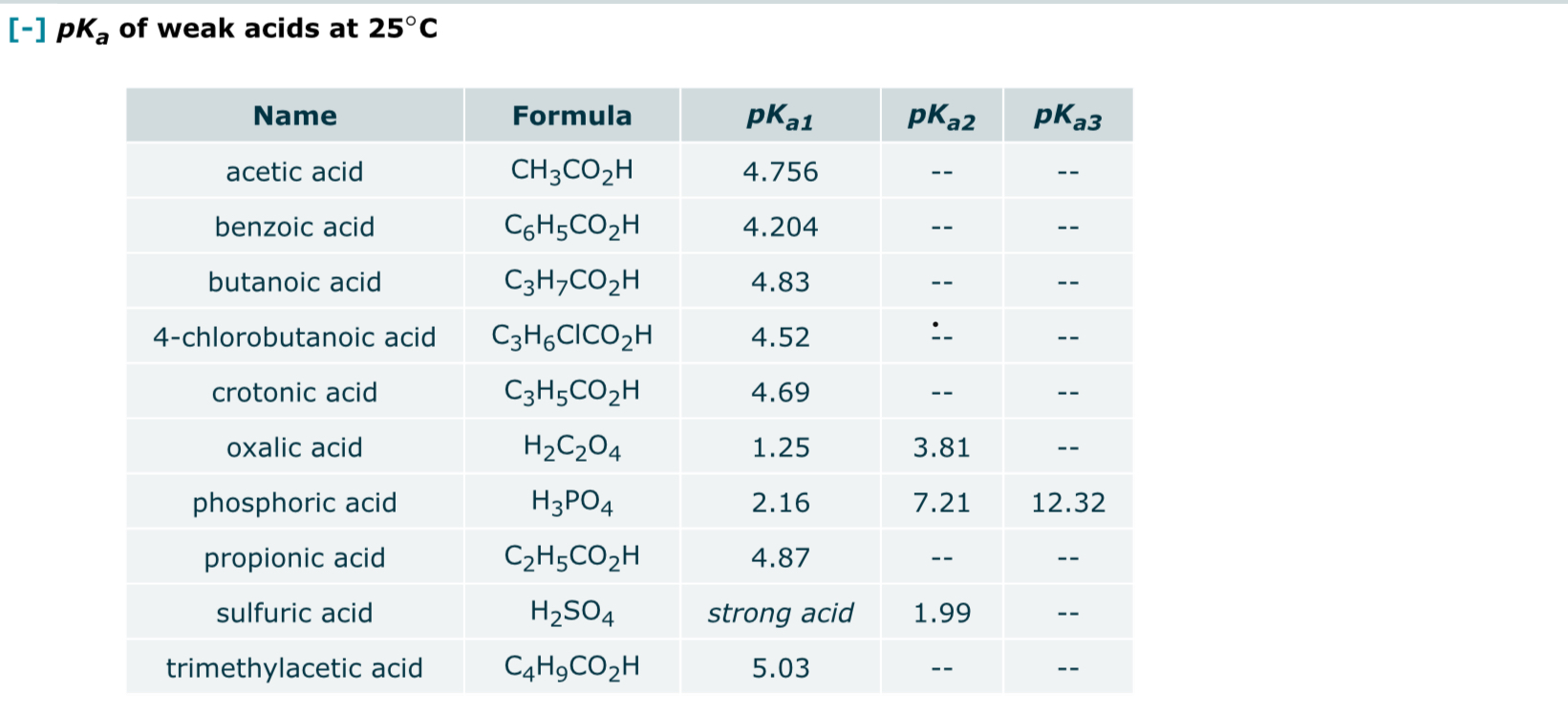
A K a for loss of the first proton is 5. Examples of molar mass computations: Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Acta Crystallographica Section E: Crystallographic Communications. Journal of Computational Chemistry. Therefore, this compound is a base. The name is also used for any bioxalates, acid oxalates, or monobasic oxalates. These materials exhibit extended structures resulting from extensive hydrogen bonding and anion-cation interactions.
Molar mass of H2C2O4(aq)
Therefore, this compound is a base. Tomato also contains another essential acid, ascorbic acid, which is better known by its common name: vitamin C. AsH3 is a stronger acid than HBr because As is larger than Br. There are two acidic protons in oxalic acid. Pathology — Research and Practice. Examples of molar mass computations: Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. H2C2O4 is a stronger acid than HC2O4- because it has more acidic hydrogens.