Ionization trend refers to the tendency of elements in the periodic table to form ions, or atoms that have a net positive or negative charge due to the loss or gain of electrons. The ionization trend is an important concept in chemistry, as it helps to predict the chemical properties and reactivity of elements.
There are several factors that influence the ionization trend of elements in the periodic table. One important factor is the atomic number of the element, which is a measure of the number of protons in the nucleus of an atom. Elements with higher atomic numbers tend to have a higher ionization energy, which is the energy required to remove an electron from an atom. This is because the protons in the nucleus of an atom are positively charged, and they exert a strong attractive force on the negatively charged electrons in the outermost energy level. As the number of protons in the nucleus increases, so does the strength of this attractive force, making it more difficult to remove an electron.
Another factor that influences the ionization trend is the electron configuration of an element. Elements with a full outer energy level, or valence shell, tend to have a lower ionization energy because they are more stable and less likely to lose or gain electrons. On the other hand, elements with an incomplete valence shell tend to have a higher ionization energy because they are less stable and more prone to losing or gaining electrons in order to achieve a stable electron configuration.
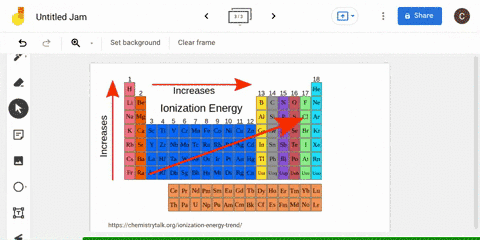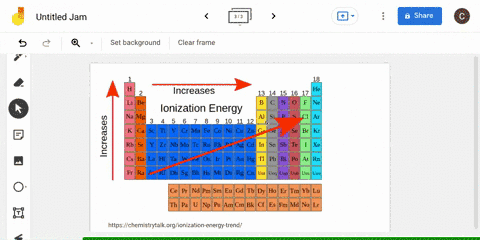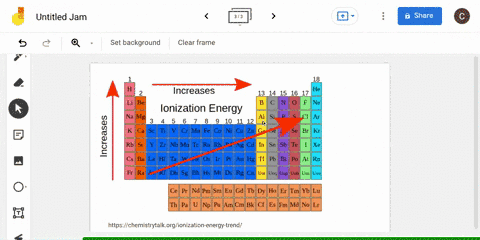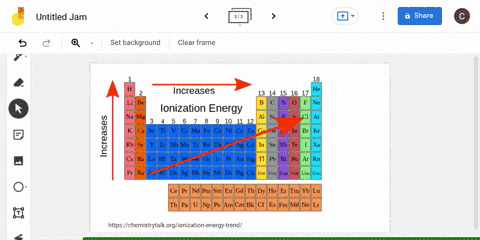
The periodic table is arranged in order of increasing atomic number, so it is possible to predict the ionization trend of elements based on their position in the table. Generally, elements in the upper left corner of the periodic table have a high ionization energy, while elements in the lower right corner have a low ionization energy. This trend is known as the "periodic trend in ionization energy," and it is a useful tool for predicting the chemical properties and reactivity of elements.
In conclusion, the ionization trend is an important concept in chemistry that refers to the tendency of elements in the periodic table to form ions. The ionization trend is influenced by the atomic number and electron configuration of an element, and it can be used to predict the chemical properties and reactivity of elements. Understanding the ionization trend is essential for predicting the behavior of elements in chemical reactions and for understanding the properties of compounds.
Ionization energy

The second ionization energy is the energy needed to remove a second electron from the energy level. In general, first ionization energies increase toward the top right corner of the periodic table, with The Octet Rule According to the How does the Period Trend Across a period, ionization energies increase. This generates a current which is proportional to the energy needed to ionise the atoms - their ionisation energy. Across a period on the periodic table, ionization energy increases with increasing atomic number with some exception, due to increasing effective nuclear charge on the outer electrons, as more electrons are added to the same shell. These factors negate the impact of increased nuclear charge.
What is the trend in ionization energy going across a period?

PDF Chemquest 22 Answer Key Ionization Trends Chemquest 22 answer key ionization trends Like many other animals, sheep travel in herds. Now you might be knowing the If we move across the period from left to right , then the atomic size decreases. Retrieved December 7, 2020. Unlike other Group 10 elements, palladium has a higher ionization energy than the preceding atom, due to its electron configuration. Such an approximation is acceptable as long as there is no multiphoton resonance between the ground state and some excited states. Horizontal arrangement of chemical elements in periodic table is termed as period.
Ionization
:max_bytes(150000):strip_icc()/GettyImages-585288171-5c49df78c9e77c0001d89abf.jpg)
It takes energy to remove electrons because they are strongly attracted to the atom's positive nucleus, which is why values for ionisation energy are positive. This anomaly is due to the fact that gadolinium valence d-subshell borrows 1 electron from the valence f-subshell. So, removing an electron becomes harder and harder, and the ionization energy increases, as atoms approach an octet. The force of repulsion between electrons. The lower the ionisation energy, the more readily an atom loses this electron to form a positive ion. There are more protons in atoms moving down a group greater positive charge , yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus.
Ionization Energy Definition and Trend
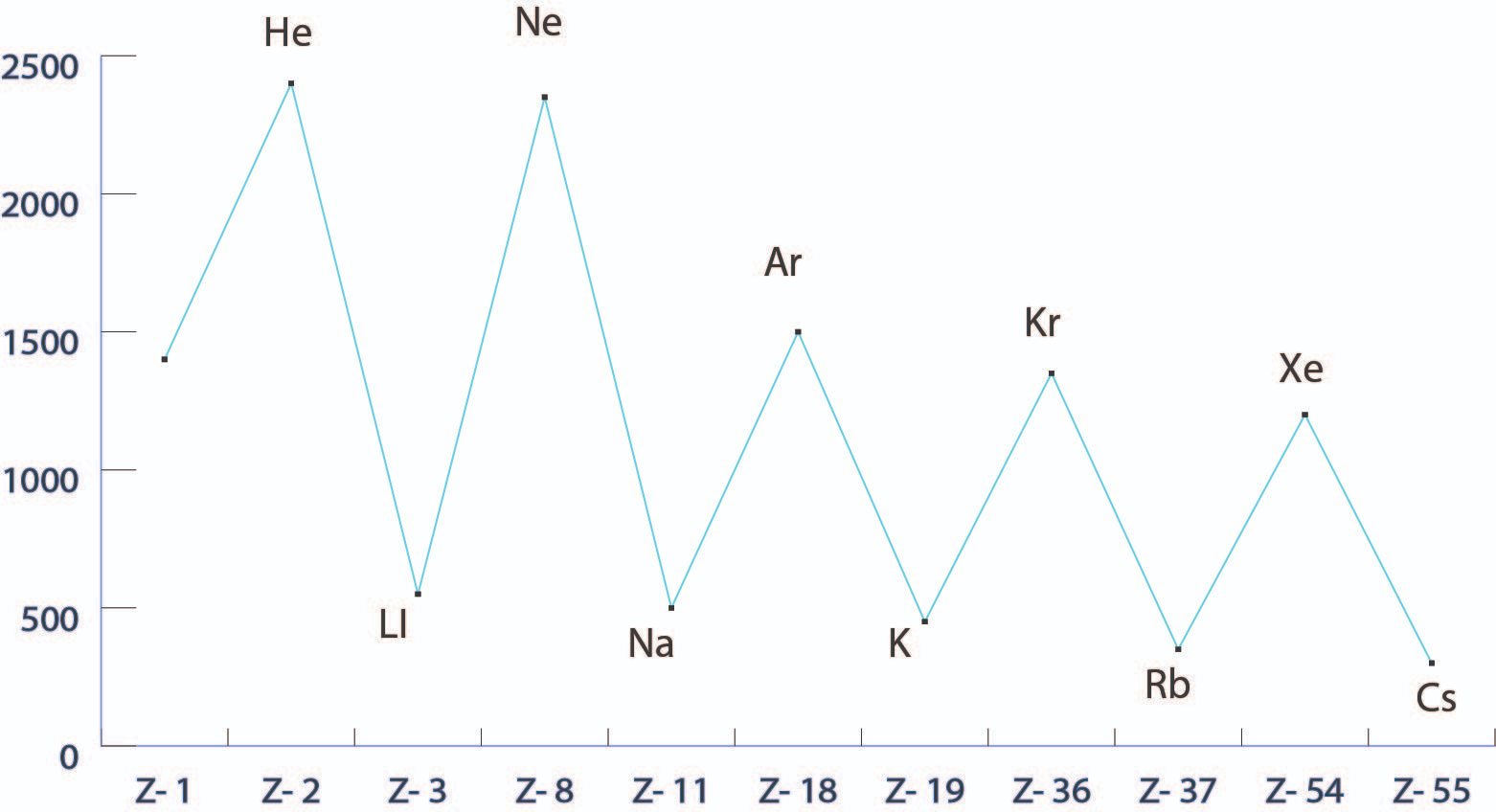
The greater the atomic radius, the less energy is necessary to pry the electron from the outermost orbital or The electron orbitals of atoms are separated into different shells. So what is the definition of ionization energy? It is the energy required to pluck out the first electron from the atom. Additionally, there are no half-filled nor fully filled orbitals we might compare. Ionization energy increases across a period due to increasing nuclear charge which leads to outer electrons being held onto very tightly. However, due to experimental limitations, the adiabatic ionization energy is often difficult to determine, whereas the vertical detachment energy is easily identifiable and measurable. The trend in effective nuclear charge accounts for the increase in ionization energy across a period.

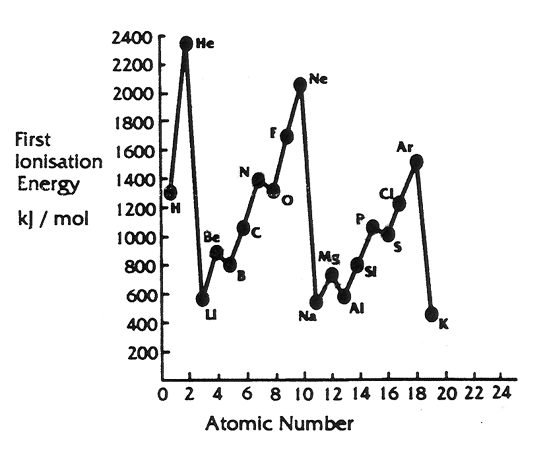


:max_bytes(150000):strip_icc()/GettyImages-585288171-5c49df78c9e77c0001d89abf.jpg)