Isopentyl acetate, also known as banana oil, is a colorless liquid with a sweet, fruity aroma. It is a common fragrance and flavor compound found in a variety of consumer products, including perfumes, candles, and flavors for food and beverages.
In terms of its chemical structure, isopentyl acetate is an ester, which is a type of compound formed by the reaction of an alcohol and a carboxylic acid. In this case, the alcohol is isopentanol, and the carboxylic acid is acetic acid. The esterification reaction between these two compounds produces isopentyl acetate and water as byproducts.
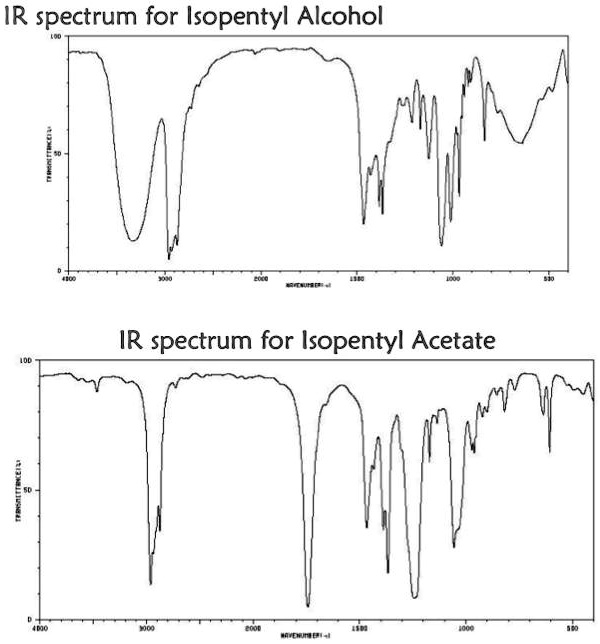
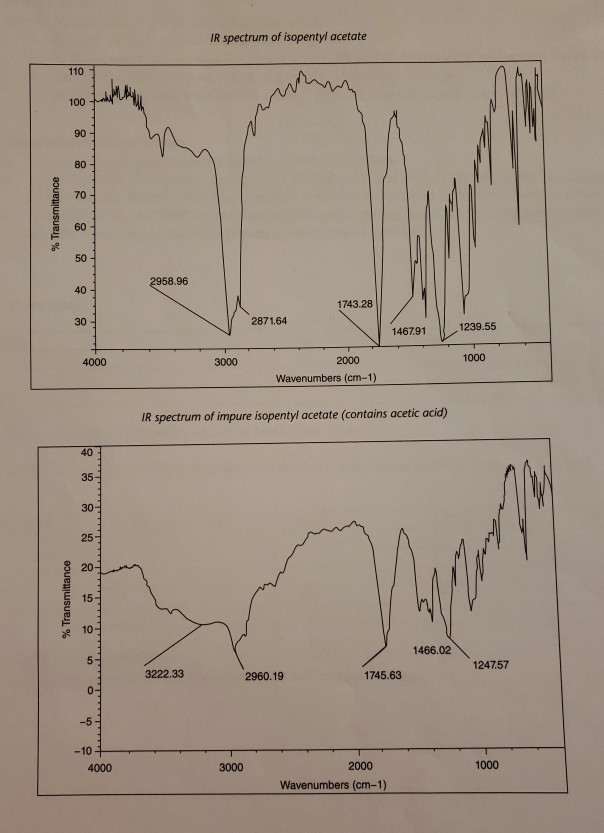
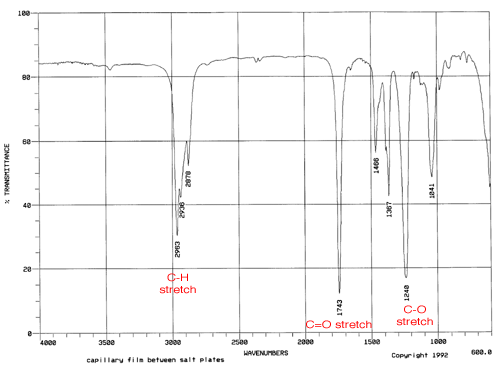
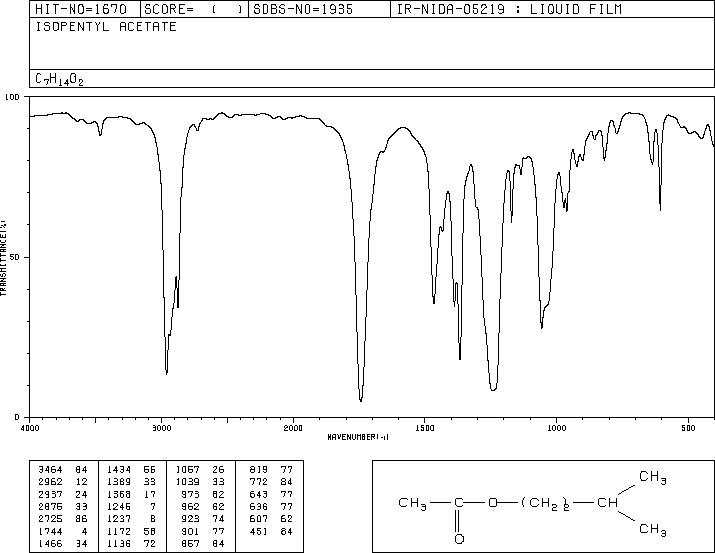
One of the key characteristics of isopentyl acetate is its infrared (IR) spectrum. IR spectroscopy is a technique used to identify and study the chemical bonds in a compound by measuring the absorption of infrared radiation by the compound. Each type of chemical bond absorbs IR radiation at a specific range of wavelengths, which allows scientists to use the IR spectrum of a compound to identify the types of bonds present and the functional groups in the molecule.
The IR spectrum of isopentyl acetate shows several key features that can be used to identify and characterize the compound. One of the most prominent features is the absorption of IR radiation at around 1740 cm-1, which corresponds to the stretching of the C=O bond in the acetate group. This absorption is strong and sharp, indicating the presence of a well-defined C=O bond in the compound.
Other key features of the IR spectrum of isopentyl acetate include absorptions at around 2920 cm-1 and 2850 cm-1, which correspond to the stretching of the C-H bonds in the isopentyl group. These absorptions are also strong and sharp, indicating the presence of well-defined C-H bonds in the compound.
Overall, the IR spectrum of isopentyl acetate is a valuable tool for identifying and characterizing this compound, and it can be used in a variety of applications, including the analysis of consumer products and the synthesis of new compounds.