ASA (American Sociological Association) is a professional organization for sociologists that promotes the advancement of sociology as a scientific discipline and serves as a resource for professionals in the field. As such, ASA has established a set of guidelines for writing and formatting sociological research papers and essays that are known as the ASA style.
The ASA style is a widely used citation and formatting style in the field of sociology and social sciences. It is similar to the APA (American Psychological Association) style, but there are some important differences. One of the key differences is that ASA style requires the use of parenthetical citations in the text of the paper, rather than footnotes or endnotes. In addition, ASA style requires a specific format for the reference list at the end of the paper.
When writing a research paper or essay in ASA style, it is important to follow the guidelines for formatting and citation. This includes using appropriate margins, font, and font size, as well as properly citing sources in the text and in the reference list.
One of the key elements of ASA style is the use of parenthetical citations in the text of the paper. This means that when you refer to a source in your paper, you include the author's name and the year of publication in parentheses at the end of the sentence. For example, "According to Smith (2020), sociological research has shown that social media use has a significant impact on people's relationships and communication patterns."
Another important aspect of ASA style is the reference list at the end of the paper. This list should include all of the sources that you cited in the text of your paper, and should be organized alphabetically by author's last name. Each entry in the reference list should include the author's name, the year of publication, the title of the work, and the publication information.
Overall, ASA style is a useful tool for writers in the field of sociology and social sciences, as it helps to ensure that research papers and essays are properly formatted and that sources are properly cited. By following the guidelines for ASA style, writers can ensure that their work is professional and scholarly, and that it adheres to the standards of the discipline.
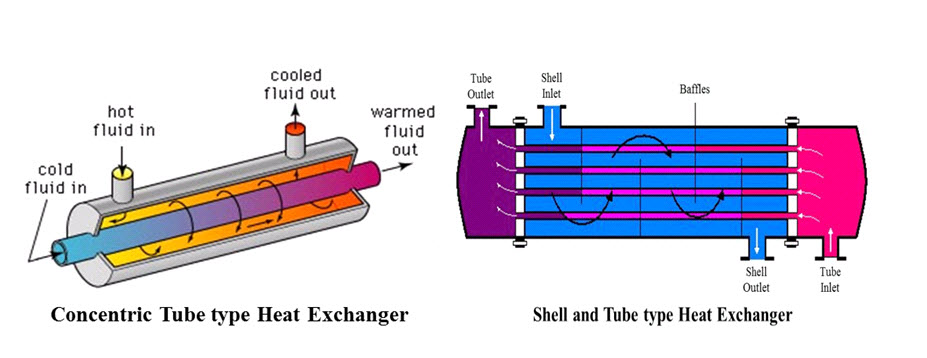
Thermal Property of Food

As the pressure is reduced, the boiling point decreases, and the latent heat value increases. At atmospheric pressure, water boils at 100 ̊C and the latent heat of vaporisation is 2257 kJ kg-1. Heat exchangers are commonly used for the purposes of heating and cooling foods. Because it takes more energy to convert a substance from one physical state to another solid to liquid, or liquid to gas , those transitions require a larger amount of energy. Do you know why does a pressure cooker cooks faster than an ordinarily covered pot? Following the comparison of the calculated Lf from the experiment with the actual value Lf of milk, which is 2. Both sensible and latent heats are observed in many processes while transporting energy in nature.
LATENT HEAT OF FUSION FOR MILK EXPERIMENT
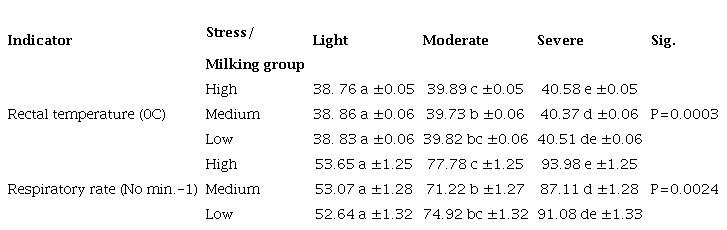
Using the specific heat value of the unknown metal and its density, it was determined that the unknown metal was Tin. A schematic DSC thermogram showing an endothermal, first-order phase transition, for example, melting. DTA measures temperature of a sample and a reference as a function of temperature. Here, we will learn about latent heat, different types of latent heat along with the formula and dimension of latent heat. . This information is significant in proving that the mass of the object is not an impactful factor in the amount of heat given off or the duration of the combustion of the object.
Latent Heat

During vaporization, the energy obviously no longer benefits the increase of the kinetic energy of the molecules, which would otherwise mean an increase in temperature see also article Figure: Vaporization of water in a pot on a hotplate During vaporization, the transferred energy leads to an increase in the broken by the added heat energy, thus allowing the transition to the gaseous state. . Conclusion: The data obtained from the experiment was able to give an approximate value for the latent heat of fusion for milk. If the amount of heat needed for a phase change is 300 kcal, calculate the latent heat of a 5 kg material. The freezing point of milk is of considerable interest, because it is also used to detect any dilution of the milk. The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization. The second error was the equipment used.





+[Higher+Level].jpg)