Liquid diffusion coefficient. blog.sigma-systems.com DIFFUSION COEFFICIENT 2022-12-17
Liquid diffusion coefficient
Rating:
7,7/10
514
reviews
Great oral topics can range from informative and educational to entertaining and controversial. The key to a great oral presentation is to choose a topic that is interesting and engaging to your audience, while also being well-researched and thoughtfully presented.
One great oral topic could be a historical event or figure. This could include a speech about a significant event in world history, such as the signing of the Declaration of Independence or the fall of the Berlin Wall. It could also include a biographical sketch of a notable figure, such as Martin Luther King Jr. or Mahatma Gandhi. These types of topics can be both informative and inspiring, as they provide a chance to learn about and reflect on important moments and individuals from the past.
Another great oral topic could be a current event or issue. This could include a discussion of a political or social issue, such as immigration reform or climate change. It could also include an analysis of a current event, such as the COVID-19 pandemic or the Black Lives Matter movement. These types of topics can be both thought-provoking and timely, as they allow for the exploration of important issues that are affecting the world today.
A third great oral topic could be a personal or creative project. This could include a presentation about a creative work, such as a painting or a short story. It could also include a discussion of a personal experience, such as a gap year or a volunteer trip. These types of topics can be both engaging and inspiring, as they allow the speaker to share their unique perspective and experiences with the audience.
Overall, great oral topics should be engaging, well-researched, and thoughtfully presented. By choosing a topic that is interesting and meaningful to both the speaker and the audience, a great oral presentation can be both informative and inspiring.
DIFFUSION COEFFICIENT

As mentioned above, the largest entropy is from mixing the multicomponent with equimolar atomic fraction, and the high entropy achieved supposedly causes the liquid phase to become more disordered, or have less local ordering, and, thus, more stable. As shown in , the superheating melting point and the crystallization temperature for the quinary HCMCA system are lower than those for the quaternary system and these values for both the systems are lower than those of pure Ni. The diffusion constants D of both the system and each individual alloy component are obtained by using the Einstein relation and the velocity—velocity autocorrelation function. SI and Imperial units. The diffusion coefficient is a physical quantity which is remains constant. Diffusion Coefficient: The term diffusion coefficient can be described as, the parameters proportionality factor which is constant between the molar flux by the diffusion of the molecular and gradient for diffusion or driving force. It is analogous to the property of thermal diffusivity in heat transfer: 1 so.
Next
Diffusion Coefficient and Temperature: Relationship and Impacts:
Today 19 6 , 349— 362 2016. Diffusion is the movement of a substance from an area of high concentration to an area of lower concentration. The physical constant factor is dependent upon some physical properties such as pressure, temperature, diffusing substance and size of the molecules. The three types of diffusion are — simple diffusion, osmosis and facilitated diffusion. Sutherland has made a correction to Eq. The cutoff distance is between the fifth and sixth nearest neighbors.
Next
Diffusion coefficient in liquids

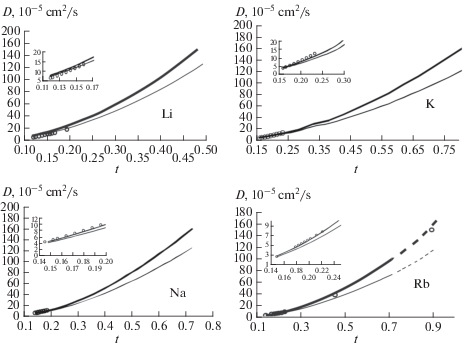
What affects the diffusion coefficient? If you weigh equal amounts or volumes of two different liquids, the liquid that weighs more is more dense. The effect of cooling rate on solidification is shown. Therefore, the activation energy obtained from the long time limit in diffusion, as defined by the Einstein relation and the velocity autocorrelation functions, is the time or ensemble average of many of these configurations. Understand how to responsibly use this work by visiting. In this work, a total of 32 000 atoms are used and the typical MD run time is about 10 ns to avoid the finite size effect and short time limitations in acquiring the diffusion constants.
Next
Experiment on Liquid Diffusion Coefficient

Only emails and answers are saved in our archive. B 49 5 , 3109— 3115 1994. K is a measure of the solubility of the substance in lipids. In metallic materials, the addition of alloy elements can effectively change the microstructures to smaller and more dispersive grains that have better overall properties, or simplify processing in solidification and subsequent heat treatment. Today 19 6 , 349— 362 2016. Temperature: A physical quantity which can be describe as the degree for a substance which could be stay in hot or cold condition.
Next
Gases Solved in Water

Thinkswap has partnered with Turnitin to ensure students cannot copy directly from our resources. These properties are known to contribute to a smaller activation energy Q and a large diffusion entropy or larger D 0. Note that the differences in the activation energies among the components are relatively small, typically within 3. The same happens in the diffusion in liquids but in a much more dynamic fashion: the atoms would spend some time executing local motion with a characteristic time that is, in general, short and varies largely depending on the temperature. Solids 49, 863— 871 1988. The inset is the illustration of two typical atomic configurations around a central atom in green and its neighbors marked as 1, 2, …, 6 at different time t. In liquids, due to the fast dynamics, the environment that each atom sees changes quickly.
Next
What is liquid diffusion?

Sherif, Phase Transformations in Metals and Alloys, 4th ed. Can a gas diffuse in liquid? Increasing temperature increases the diffusion coefficient, as demonstrated by the equation relating the diffusion coefficient to temperature. The same occurs as molecule mass and size increase. China 16, 1228— 1235 2006. Thus, as compared to crystals, the activation energy that a liquid atom experiences is an average of many of these dynamically changing environments. Temperature Image Credit — Diffusion coefficient and temperature relationship: From the The diffusion coefficient and temperature relationship is directly proportional to each other means if the coefficient of diffusion is increases then the temperature is also gradually increases as well as if the value of coefficient of diffusion is decreases then the temperature is also decreases. For a high-viscosity solvent, they are in great error and therefore inapplicable.
Next
blog.sigma-systems.com DIFFUSION COEFFICIENT
. Our work reveals that it is the increasing disorder induced by the increasing number of alloy components that leads to the maximum entropy. By the help of thermometer temperature is measure. It is also inversely proportional to the square root of density. What is self diffusion coefficient? In aqueous the liquid substance -9 to 10 -8 square meter per second.
Next
Homogenization of diffusion in multicomponent liquids: The Journal of Chemical Physics: Vol 157, No 24
The experimental data of Wilke and Chang give available evidence that the activation energy varies from 12. Their effects are summarized early in a set of empirical rules for MG formation 10 10. The activation energies for all the alloy elements with different bonding energies and atomic sizes are close to each other. Steam - Critical and Triple Points - Critical point is where vapor and liquid are indistinguishable and triple point is where ice, water and vapor coexist in thermodynamic equilibrium. Read more about For different substances the coefficient of diffusion can be estimate from different equations. Comparison of this equation with 96 experimental points has shown good agreement, the spread of points being about +15%.
Next

Each of these factors, independently and collectively can alter the rate and extent of diffusion. But it is directly proportional to pressure. One of the earliest equations for determining the diffusion coefficient in dilute solutions was the Stokes-Einstein equation, based on the model of motion of a spherical particle of diffusing substance A in a viscous liquid continuum B where r 0 is the particle molecule radius and η B, the liquid viscosity. The heavy molecule movement in the diffusion system is very slow than the lighter molecule. Carbon dioxide and oxygen are the two gases in air which dissolves in water by diffusion. Thakar 1953 , When one or more of the The Although there are a lot of data in the literature regarding A number of useful empirical and semiempirical relations have been proposed for Equation 2 may be used for the In The Because of the low In relation 8.
Next

The collision of the ink molecules with the water molecules keeps diffusion from happening quickly. Our atomistic modeling reveals that this result is the direct consequence of random mixing of the equimolar components: the largest entropy from the mixing corresponds to the most random distribution of the alloy components. Diffusion occurs in liquids and gases when their particles collide randomly and spread out. The kinetic theory of gases makes it possible to determine the constant in Eq. A known concentration of sodium chloride solution is placed in a diffusion cell immersed in distilled water. Google use cookies for serving our ads and handling visitor statistics.
Next









