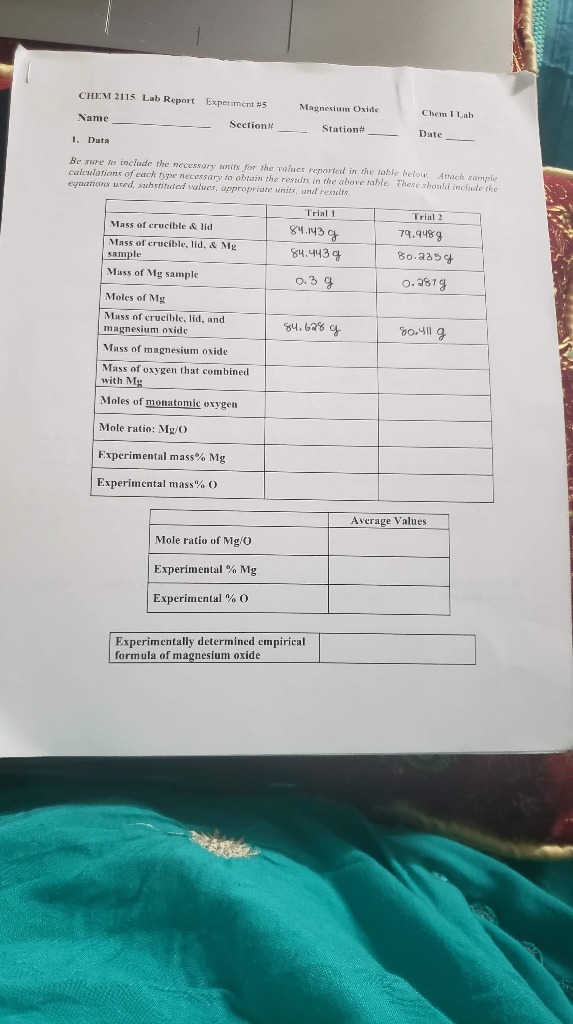
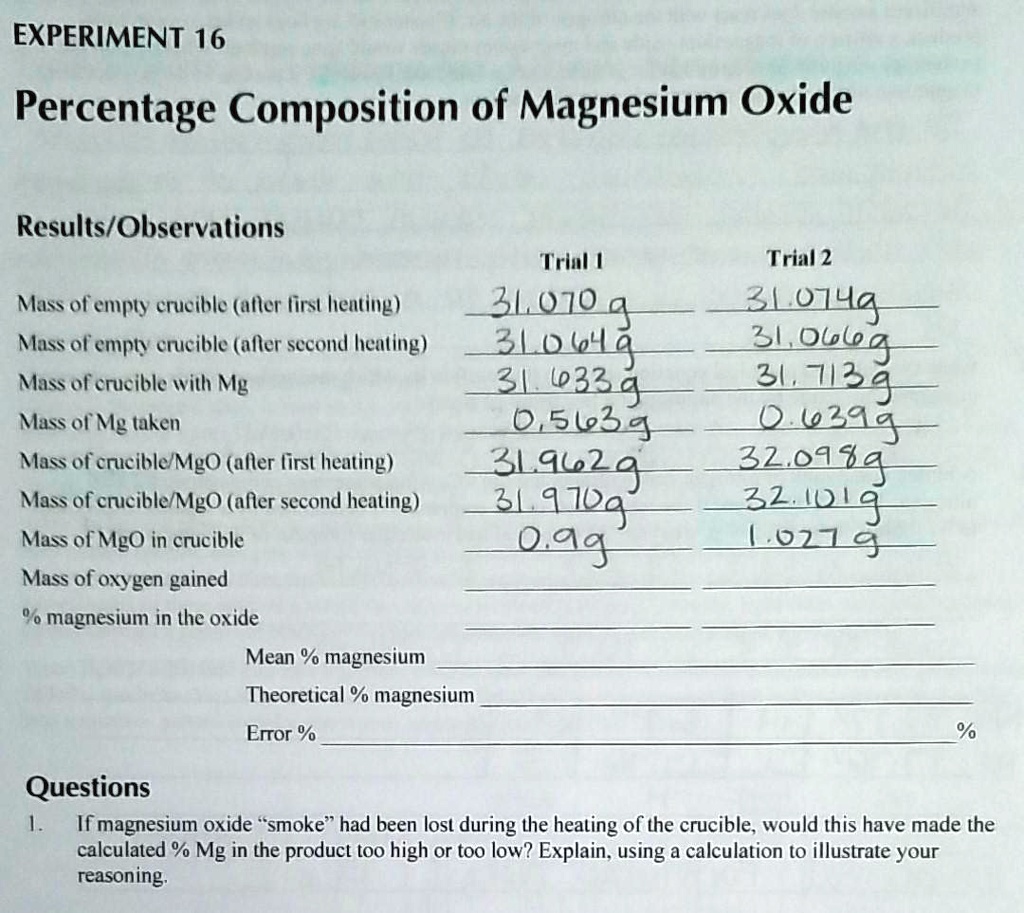
Magnesium oxide, also known as magnesia, is a chemical compound made up of magnesium and oxygen. It is a white solid substance with a high melting point and is commonly used in various industrial and medicinal applications. In this experiment, the goal was to determine the percent purity of a sample of magnesium oxide by performing a gravimetric analysis.
To begin the experiment, a sample of pure magnesium metal was weighed on an analytical balance and placed in a crucible. The crucible was then placed in a bunsen burner and heated until the magnesium had completely burned and turned into magnesium oxide. The crucible was allowed to cool, and the magnesium oxide was then weighed on the analytical balance to determine the mass of the product.
Next, the magnesium oxide sample was dissolved in hydrochloric acid, which reacts with the magnesium oxide to form magnesium chloride and water. The magnesium chloride was then filtered and washed with distilled water to remove any impurities. The purified magnesium chloride was then dried in an oven and weighed on the analytical balance to determine the mass of the pure magnesium chloride.
The percent purity of the magnesium oxide sample was then calculated using the following formula:
% Purity = (Mass of Pure Magnesium Chloride / Mass of Magnesium Oxide) x 100%
The results of the experiment showed that the sample of magnesium oxide had a percent purity of 99.5%. This indicates that the sample was relatively pure, with only a small amount of impurities present.
Overall, the gravimetric analysis of the magnesium oxide sample was a successful experiment. It provided an accurate determination of the percent purity of the sample and demonstrated the effectiveness of using gravimetric analysis as a method for analyzing the purity of a substance.
Magnesium Oxide Reaction

This relates to the magnesium oxide lab because we tested out this theory to see if it is true that the law of definite proportions says that a chemical compound contains the same element in exactly the same proportions by mass regardless of the size of the sample or source of the compound. The properties of Magnesium and reactivity was investigated into, as it helped conduct further experiments which would benefit while calculating percent yield. Weigh the crucible with the lead, 15. Of note, some experimental protocols instruct students to reheat the crucible and Magnesium ribbon several times and re-measure the weight each time continuing until the final mass value is repeatedly constant. Be aware of how dangerous this chemical can be, so please be careful. This showed the reaction of Oxygen combining with Magnesium to form Magnesium Oxide.
Stoichiometry_Magnesium_Oxide_Lab_Report

The report will look to understand the percent yield of Magnesium Oxide MgO after heating varying amounts of Magnesium via a conventional Bunsen Burner or Ceramic hot plate. In this lab, the student burned magnesium, in the form of a ribbon, which bonded with oxygen to form magnesium oxide. Magnesium oxide is a compound composed of a metal and a nonmetal. After we make the oxide, we will then determine the empirical formula. To find out the empirical formulae for magnesium-oxide Introduction.
Lab Report On Magnesium Oxide

. The melting point, Infrared Spectroscopy, 13C NMR, and 1H NMR were used to characterize and confirm Pre-lab quiz for chem 243b Essay 1 point At what time do you have to complete your online prelab assignment to be allowed to participate in the lab? Design: The following figure represents the experiments lab setup, visualize the equipment used. In this case if I were to determine a systematic error during the lab I would consider making a graph which would represent the data collected during the experiment. While the balance door is closed, click the Tare button and wait for the balance to read close to zero. The reason that the product had a higher mass than the reactant is because Mg bonded to O to form MgO, so the product had a higher mass because of the gain of an O atom.