Melting point of aspirin. The melting point of aspirin : 128 2022-12-29
Melting point of aspirin
Rating:
5,4/10
1900
reviews
Aspirin, also known as acetylsalicylic acid, is a commonly used over-the-counter pain reliever and anti-inflammatory medication. One of the physical properties of a substance is its melting point, which is the temperature at which it transitions from a solid to a liquid. The melting point of a substance can be an important characteristic, as it can affect the stability and storage of the substance, as well as its suitability for certain applications.
The melting point of aspirin is approximately 136-138°C. This is a relatively low melting point compared to other substances, and it is one of the reasons why aspirin is often formulated as a tablet or capsule, rather than as a solid block or cube. The low melting point also means that aspirin can be easily dissolved in water, which is useful for creating solutions or suspensions for oral administration.
However, the low melting point of aspirin can also present some challenges. For example, if the temperature of the storage environment is too high, the aspirin may begin to melt or become deformed, which can affect its potency and stability. As a result, it is important to store aspirin in a cool, dry place, away from direct sunlight or other sources of heat.
In conclusion, the melting point of aspirin is an important physical property that can affect its stability, storage, and suitability for certain applications. It is important to consider the melting point of a substance when selecting it for a particular use or when storing it properly.
Preparation of Aspirin and Determination of the Melting...

A skin and severe eye irritant. The acid chloride needles has m 19-19. There was problem about fleeing from infested areas. The dose required for benefit appears to depend on a person's weight. Before filling a class V abrasion cavity with GIC you should A. If you took a mixed melting point where the ratio of A is higher than B, what would the melting range look like? Essay On Ibuprofen 1135 Words 5 Pages Audience: This paper is written with the intent of addressing an audience consisting of advanced high school students and lower division college students, who are interested in learning what happens to their bodies internally when ibuprofen is consumed.
Next
Salicylic acid

By 1860, organic chemists were able to synthesize salicylic acid from basic starting materials, this furthered the therapeutic use of the substance, but there were problems. The melting point of the aspirin was found to be 110-115 degress Celsius. Aspirin, an acetyl derivative of salicylic acid, is a white, crystallize, weakly acidic substance, with a melting point of 136oC and a boiling point of 140oC Meyers, 2007. Additionally, aspirin acts on prostaglandins in the hypothalamus to reset and reduce a raised body temperature. The melting points for the acidic and neutral compounds were hence too low, and the melting point for the basic compound was too high.
Next
The melting point of aspirin : 128

For centuries it has been known that willow-bark was useful for treating inflammation, pain, and fever Experiment 3, 2013. Aspirin is an oral non-steroidal anti-inflammatory drug NSAID that is rapidly absorbed from the stomach and the small intestine. Sources are considered primary, secondary, or tertiary depending on the originality of the information presented and their proximity or how close they are to the source of information. You can use RCOOH to represent aspirin. Journal of the American College of Cardiology.
Next
The Chemistry of Aspirin

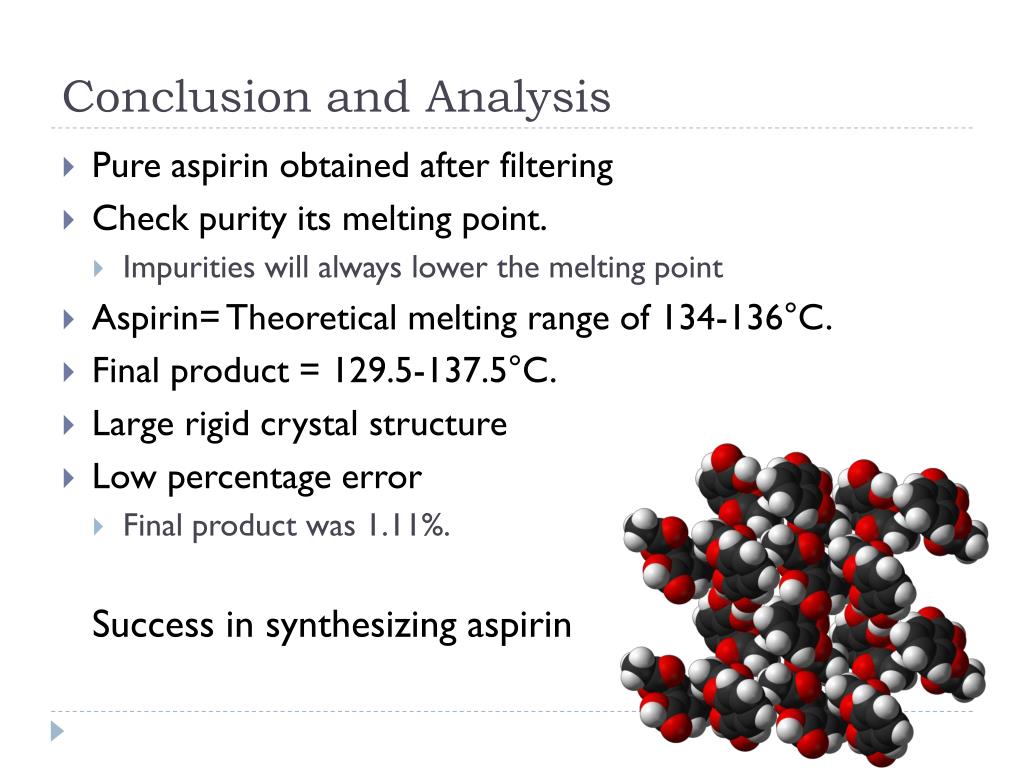
This demonstrates that the aspirin was successfully separated and is relatively pure. The insoluble portion was gravity filtered and air dried to yield 0. Salicylic acid has long been a key starting material for making acetylsalicylic acid aspirin. Salicylic acid is derived from the bark and leaves of the willow tree. Abstract: The purpose of this experiment was to determine the level of purity by using the values for melting point and absorbance and chemically synthesizing aspirin by using phosphoric acid as a catalyst. In 1897, scientists at the drug and dye firm :69—75 By 1899, Bayer had dubbed this drug Aspirin and was selling it globally. Also, when transferring the crude aspirin into the vacuum filtration, some of the crystals stayed in the flask.
Next
organic chemistry

A meta-analysis through 2019 said that there was an association between taking aspirin and lower risk of cancer of the colorectum, esophagus, and stomach. This new wonder pain killer was found in Meadowsweet Spiraea ulmaria , a wild flowering plant that grows on riverbanks over much of Europe. Since aspirins inception as a commercial product the various methods of production have been studied exhaustively to achieve the highest synthesis efficiency while also yielding the highest purity aspirin possible from the least amount of reactants, and thus reducing the final cost of the product. Aspirin is part of a group of medications called nonsteroidal anti-inflammatory drugs NSAIDs , but differs from most other NSAIDs in the mechanism of action. Since the formula for aspirin is C9H8O4, it contains 9 atoms of carbon and is therefore organic. In those with prior ischaemic stroke or acute myocardial infarction, daily low dose aspirin was associated with a 19% relative risk reduction of serious cardiovascular events non-fatal myocardial infarction, non-fatal stroke, or vascular death. Unknown Crystals 532 Words 3 Pages The Identity of the unknown, in this case unknown A, was determined to be acetyl salicylic acid.
Next
Aspirin Melting Point Analysis

Why and how it did that cannot be answered as the server-side engine is proprietary. Later the information may be summarized into an encyclopedic or reference book format tertiary sources. Cyclooxygenase-3 COX-3 : Filling in the gaps toward a COX continuum?. The polarity of the spots also determines how far it moves on the plate; non-polar spots are higher than polar ones. Indications Salicylic acid is a β-hydroxy acid that penetrates into the sebaceous gland and has comedolytic and anti-inflammatory properties.
Next
Aspirin

This sample had a median melting point temperature of 129oC. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Phenacetin Synthesis 424 Words 2 Pages The main objective of this experiment was the formation of phenacetin from the synthesis of acetaminophen. Salicylic acid, the main metabolite of aspirin, is an integral part of human and animal metabolism. After the alumina filtration step, he stopped the experiment for a week. New England Journal of Medicine.
Next
Properties
It uncouples Aspirin is readily broken down in the body to salicylic acid, which itself has anti-inflammatory, antipyretic, and analgesic effects. The Rf values of isolated aspirin and pure aspirin were the same. These include aspirin pKa 3. This has very important practical applications. Retrieved 13 July 2009. It is an acidic medicine associated with gastric irritation and acid reflux, which in turn can lead to low oral pH levels. What is the molecular weight of aspirin C9H8O4? The mass of the recrystallized aspirin was 1.
Next
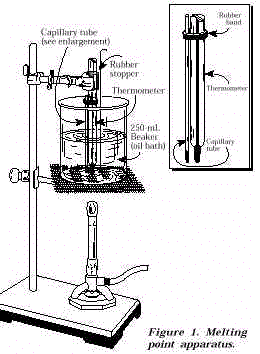
Preparation of Aspirin and Determination of the Melting Point

Ref: Hansch,C et al. This antithrombotic property makes aspirin useful for reducing the incidence of heart attacks in people who have had a heart attack, unstable angina, ischemic stroke or transient ischemic attack. It helps dissolve the top layer of stratum corneum cells, improving the look and feel of the skin. The median melting point temperature of the recrystallized aspirin was 131. And then determine the relative purity of the synthesized sample.
Next
Aspirin
It is used to help reduce pain and swelling, but there are also a few side effects that may occur. Benefits for Skin Salicylic acid is a beta hydroxy acid that also sloughs dead cell buildup within the follicle, acts as a mild antibacterial, and has soothing properties. PREPARATION OF ASPIRIN AND DETERMINATION OF THE MELTING POINT Ferrer, Lara Melissa V. Prostaglandins are found throughout the body and are made to help manage injury or infection. The melting and boiling point of the substances will help in concluding on which of these compounds will be presented at the end of experiment. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat.
Next








