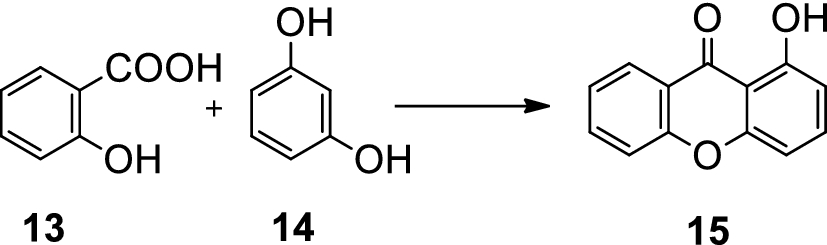
Naphthalene picrate, also known as picric acid or 2,4,6-trinitrophenol, is a highly explosive compound that is often used as a military explosive and a laboratory reagent. It is a pale yellow, crystalline solid that is highly sensitive to impact and friction, making it difficult to handle and store safely.
Naphthalene picrate was first synthesized in the late 19th century by the German chemist Adolf von Baeyer, who was attempting to create a more stable alternative to nitroglycerin, a highly explosive compound that was commonly used at the time. Naphthalene picrate proved to be a more stable and less sensitive explosive, and it was soon adopted for use in military applications.
In addition to its use as an explosive, naphthalene picrate has a number of other applications. It is often used as a laboratory reagent in the synthesis of other chemicals, and it has been used as a dye and a pigment in the past. It is also used in the production of explosives, explosives primers, and pyrotechnics.
Despite its usefulness, naphthalene picrate is a highly dangerous compound that must be handled with caution. It is highly sensitive to impact and friction, and it can ignite spontaneously if it is not stored properly. It is also toxic if ingested or inhaled, and it can cause severe skin irritation if it comes into contact with the skin.
In summary, naphthalene picrate is a highly explosive compound that is used as a military explosive and a laboratory reagent. It is a pale yellow, crystalline solid that is highly sensitive to impact and friction, and it must be handled with caution due to its potential to ignite spontaneously and its toxic nature.