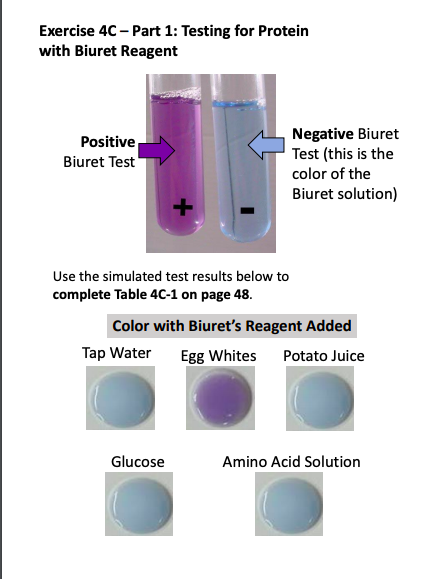
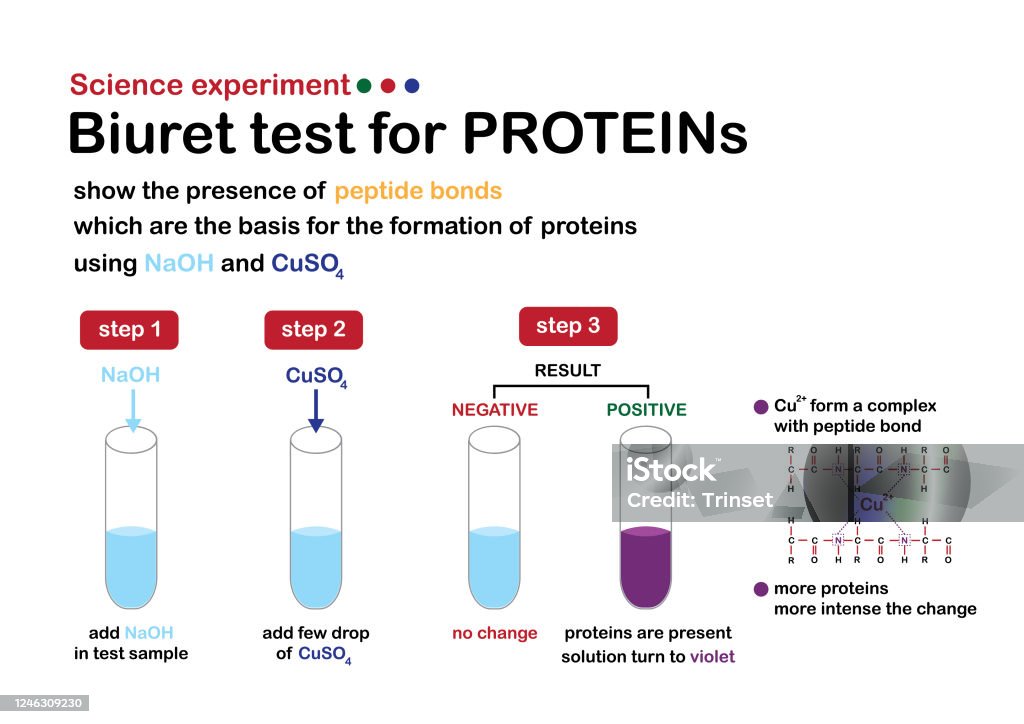
A biuret test is a chemical test used to identify the presence of proteins in a solution. It is based on the reaction of proteins with copper ions to form a purple-colored compound called biuret. The intensity of the color is directly proportional to the concentration of protein in the solution.
A negative biuret test result means that no proteins were detected in the sample. This can occur for several reasons. One possibility is that the sample simply does not contain any proteins. This can be the case with pure water or other non-biological fluids.
Another reason for a negative biuret test result could be that the protein concentration in the sample is too low to be detected by the test. The sensitivity of the biuret test varies depending on the specific protocol used, but it is generally less sensitive than other protein detection methods such as the Bradford assay or the BCA assay.
A negative biuret test result could also be the result of sample degradation or contamination. Proteins are sensitive to degradation by heat, pH, and other environmental factors, and can be easily denatured or hydrolyzed. Contamination with substances that interfere with the biuret reaction, such as detergents or surfactants, can also lead to a false negative result.
In conclusion, a negative biuret test result indicates the absence of proteins in a sample, but it is important to consider the potential causes of a false negative result and to consider using additional methods to confirm the absence of proteins.
Why does glycine give a negative biuret test?

Specifically, peptide bonds C-N bonds in proteins complex with Cu 2 + in Biuret reagent and produce a violet color. A Cu2+ must complex with four to six peptide bonds to produce a color; therefore, free amino acids do not positively react. No change in colour. Normally,biuret test can give pozitive result that means purple color with proteins. Such ions remain electrically neutral and therefore do not travel in an electric field, since they possess one positive as well as one negative charge. Biuret reagent is an alkaline solution of 1% CuSO4, copper sulfate.
Why would amino acids give a negative result in the biuret test for proteins?
The copper II present in the biuret reagent binds itself to the nitrogen atoms that are present in the protein peptides. Once this complex has been formed, the solution turns from a blue color to a purple color. The blue colour will change to violet if protein is present. Biuret Test for Various Substances The casein and pure protein content of skimmed milk, as well as whole milk's protein content, can be measured with the help of Biuret test solutions which consist of potassium hydroxide and a detergent. So, what distinguishes a peptide from a protein? Add 1 mL of 40% NaOH solution and 1 or 2 drops of 1% CuSO4 solution.
Which is the biuret test? Explained by FAQ Blog
When a biuret test is positive What color does the solution turn? It is a white solid that is soluble in hot water. It demonstrates a negative test result no protein present. What is the color change for protein? The false-positive test result can also be observed when diamides of oxalic, malonic and succinic acid are present. If protein is not present, the blue colour will remain. This chelate complex has the ability to absorb light with a wavelength of 540nm, which imparts a purple color to it. The Biuret test for protein is useful in the case of both cheese and meat as they get dissolved in potash lye or solutions of alkaline detergent.
Why would amino acids give a negative result in the biuret test for proteins?

How is the biuret test used to check for peptide bonds? A Cu2+ must complex with four to six peptide bonds to produce a color; therefore, free amino acids do not positively react. The test is named so because it also gives a positive reaction to the peptide-like bonds in the biuret molecule. Histidine is the only amino acid that gives Biuret test positive. The test, however, gives positive result to any compound containing two carbonyl groups attached to a nitrogen or carbon atom. Only amino acid, Histidine, gives a positive result. Traditionally, peptides are defined as molecules that consist of between 2 and 50 amino acids, whereas proteins are made up of 50 or more amino acids.
What causes the color change in biuret test?

Proteins are basically different amino acids that are connected by peptide bonds. The polypeptide chain is a chain of amino acids linked together by peptide bonds. Increasing Biuret Test Sensitivity Cu+ is a strong reducing agent that can react with Mo VI during Folin-Ciocalteu's test to produce molybdenum blue. What is violet colour in Biuret test due to? This turns a mauve or purple colour when mixed with protein. Also Visiting Faculty of: Central Department of Microbiology Tribhuvan University TU , Nepal , Central Department of Biotechnology Tribhuvan University TU , Nepal , Amrit Science Campus ASCOL Kathmandu, Nepal. What is the purpose of negative control? An egg white before the denaturation of the albumin protein causes the transucent substance to change in color and viscosity. The determination of lower levels of proteins requires more sensitive methods.
Biuret Test

Iodine solution IKI reacts with starch to produce a dark purple or black color. Also, it is less expensive than the Kjeldahl test. Biuret reagent is an alkaline solution of 1% CuSO4, copper sulfate. Why would amino acid give a negative result in the Biuret test for protein? As 2 peptide bonds are required for the formation of the chelate complex, single amino acids — no peptide bonds present — and dipeptides — only 1 peptide bond present — give a negative result. Hence a color change from blue to violet indicates that proteins are present. What danger does Benedicts solution pose to your skin? Know more about it here. The reason behind this colour is the formation of a chelate complex or the copper coordination complex.
What does a negative biuret test indicate?

Beside above, do all proteins give a positive result to biuret test? The alkaline environment is supplied by sodium hydroxide and potassium hydroxide. An example is the ninhydrin test in which the amine functional group of α- amino acids reacts with ninhydrin to form purple-colored compounds. Biuret reagent is a solution of potassium sodium tartrate, sodium hydroxide NaOH , or potassium hydroxide KOH. Also, to ensure that the test sample is alkaline, add a few drops of 5% sodium hydroxide solution to each test tube. Why is a positive and a negative control used for each biochemical test? As a matter of fact, the presence of excess protein in urine can result in kidney diseases and other complications like high pressure, diabetes mellitus, etc.