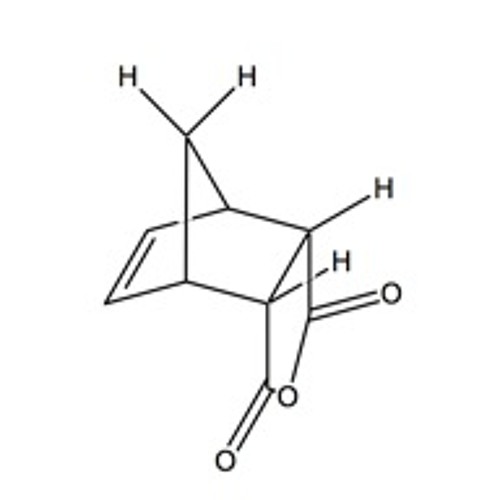
Norbornene dicarboxylic anhydride (NDCA) is a chemical compound that belongs to the class of organic compounds known as norbornenes. It is a cyclic compound with a seven-membered ring structure and is composed of two carboxylic acid groups and an anhydride group. NDCA is a white solid at room temperature and has a melting point of around 174°C.
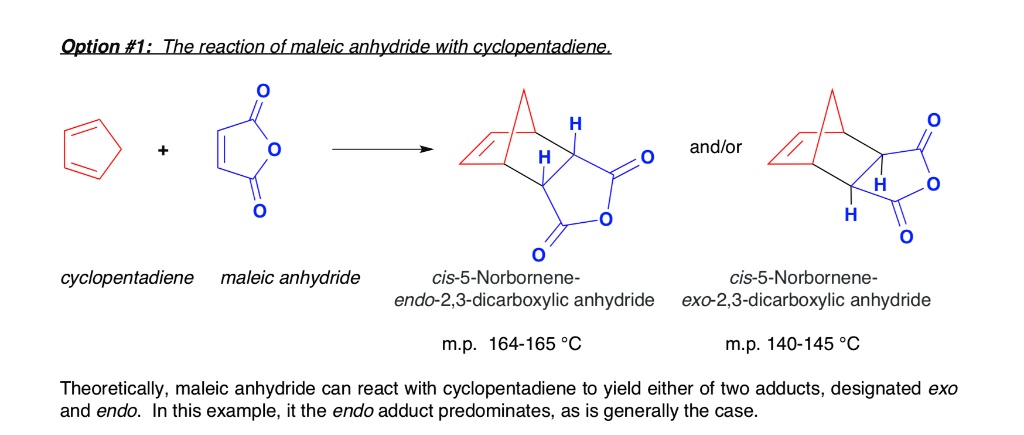
NDCA is synthesized through the dehydration of a dicarboxylic acid, such as maleic acid, using a strong acid catalyst. It can also be synthesized through the Diels-Alder reaction between norbornadiene and maleic anhydride. The resulting product is a mixture of isomers, including cis- and trans-NDCA.
NDCA has a number of important industrial applications. It is used as a monomer in the synthesis of polymers, such as polyamides and polyimides, which have a wide range of applications including coatings, adhesives, and fibers. NDCA is also used in the production of resins, plasticizers, and rubber additives.
In addition to its use in the polymer industry, NDCA has also been studied for its potential use in the pharmaceutical industry. It has been shown to have anti-inflammatory and analgesic properties, and it has been suggested that it may have potential as a drug for the treatment of pain and inflammation.
Despite its potential benefits, NDCA also has some potential drawbacks. It is a known irritant to the skin and eyes, and it may also be harmful if ingested or inhaled. Therefore, it is important to handle NDCA with caution and to use appropriate protective equipment when working with it.
In conclusion, norbornene dicarboxylic anhydride is a versatile chemical compound with a number of important industrial and potential pharmaceutical applications. However, it is important to handle it with caution due to its potential irritant and harmful effects.
129
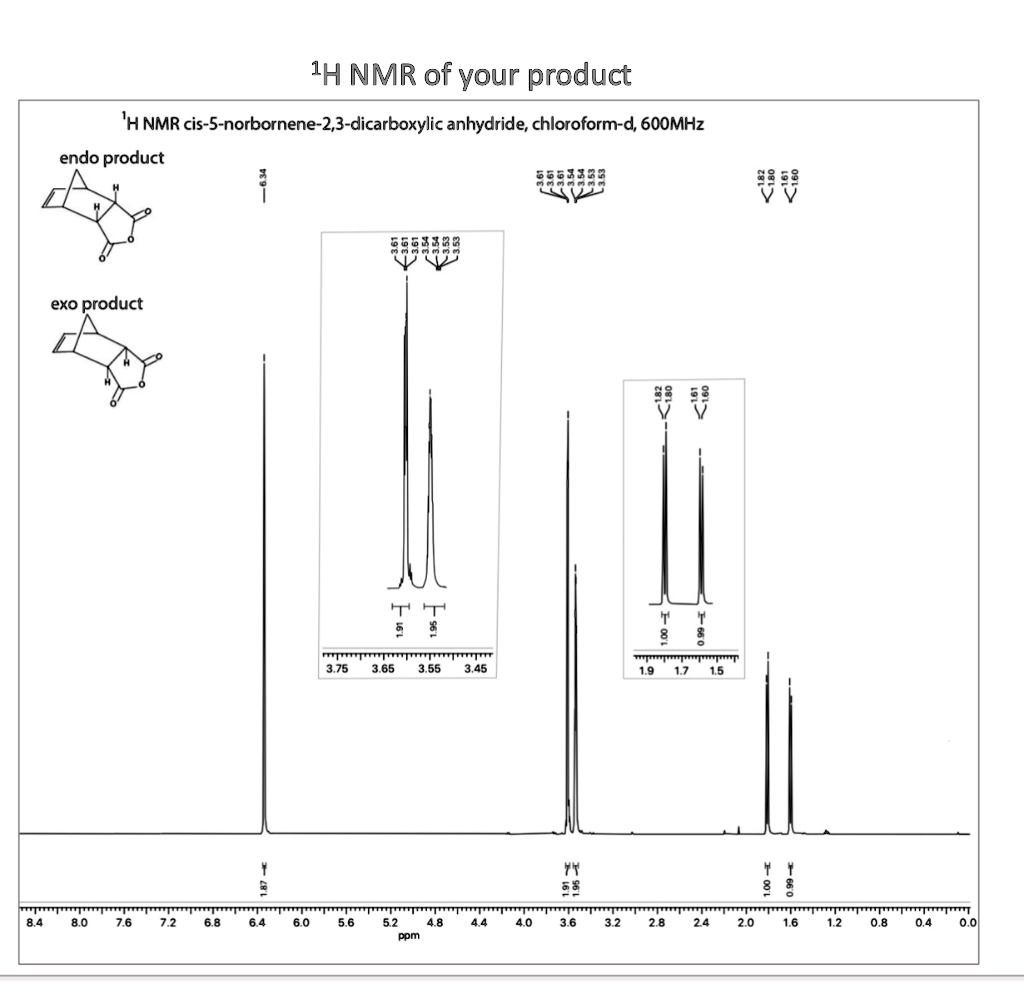
Do NOT induce vomiting P302 + P352 - IF ON SKIN: Wash with plenty of soap and water P304 + P340 - IF INHALED: Remove person to fresh air and keep comfortable for breathing P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. The NMR spectrum of the copolymer in acetone-d 6 at room temperature showed absorption in the 5. P285 : In case of inadequate ventilation wear respiratory protection. We may terminate your access, or suspend any user's access to all or part of the Site, without notice, for any conduct that we, in our sole discretion, believe is in violation of any applicable law or is harmful to the interests of another user, a third-party provider, a service provider, or us. The NMR spectrum contained peaks centered at 6.
Influence of norbornene dicarboxylic anhydride on the copolymerization of carbon dioxide and propylene oxide

The free radical precursor is used in catalytic quantities, e. Harvest or otherwise collect information about others, including e-mail addresses, without their consent. A process for the preparation of terpolymers of maleic anhydride and the exo and endo cyclic adducts of maleic anhydride and a cyclic conjugated diene, said diene selected from the group consisting of cyclopentadiene and methylcyclopentadiene, which comprises heating maleic anhydride and either cyclic adduct in the presence of a free radical precursor, at a reaction temperature which is a temperature of endo-exo isomerization of the adduct. If you choose to follow any such recommendation you do so at your own risk. P363 : Wash contaminated clothing before reuse. The Diels-Alder adduct from cyclopentadiene and maleic anhydride, endo-cis-5-norbornene-2, 3-dicarboxylic anhydride, m. We have become experts in scientific operations, improving performance with sophisticated solutions and providing guidance on best practices.