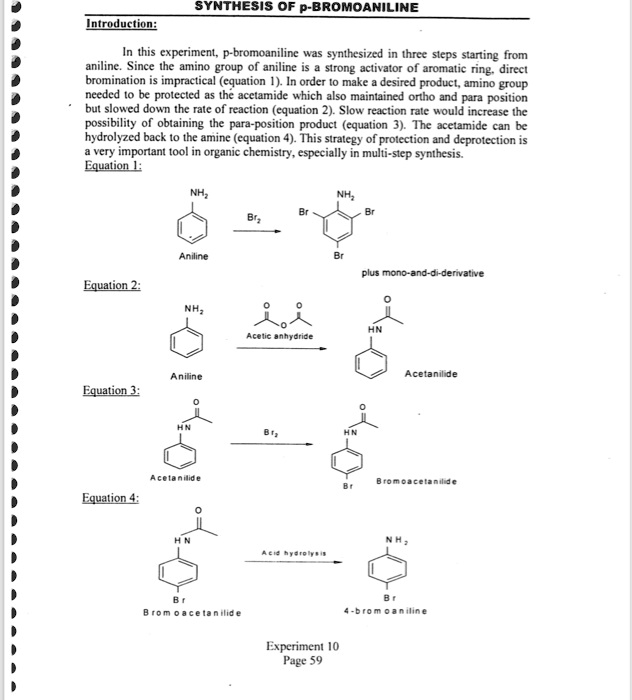
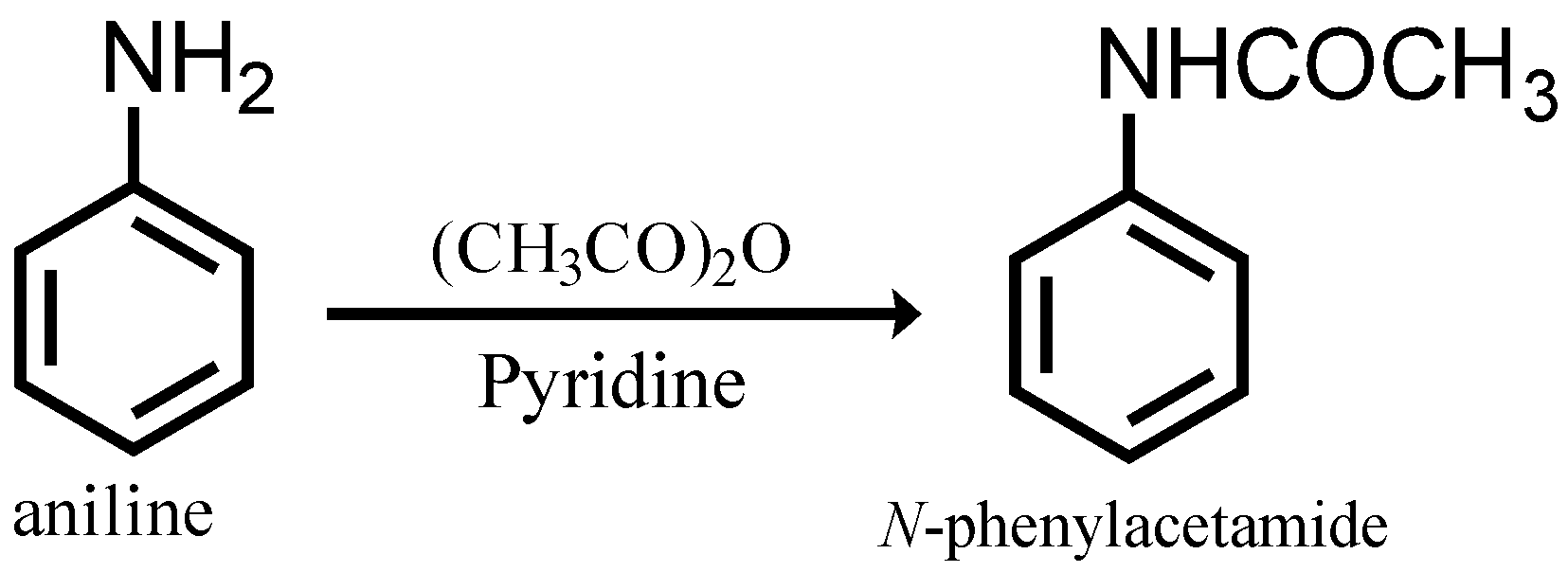
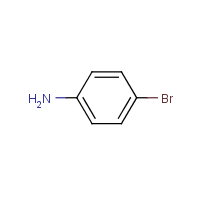
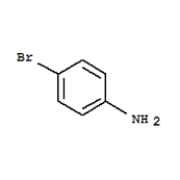
P-bromoaniline is an organic compound that belongs to the class of compounds known as amines. It is a derivative of aniline, which is a primary aromatic amine. P-bromoaniline is a colorless liquid with a pungent, fish-like odor. It is miscible with water, alcohol, and ether, but insoluble in hydrocarbons.
P-bromoaniline is used as an intermediate in the synthesis of a variety of chemicals, including dyes, pharmaceuticals, and agrochemicals. It is also used as a starting material for the synthesis of p-bromoacetanilide, which is used as a herbicide.
P-bromoaniline is produced by the bromination of aniline, which is a reaction between aniline and bromine. The reaction is carried out in the presence of a solvent, such as water or alcohol, and a catalyst, such as sulfuric acid. The resulting product is a mixture of p-bromoaniline and m-bromoaniline, which can be separated by distillation.
P-bromoaniline is a hazardous chemical and should be handled with caution. It is classified as a dangerous good and should be transported, stored, and used in accordance with the relevant regulations. It is toxic by ingestion and inhalation, and can cause skin and eye irritation. It is also a flammable liquid, and should be stored in a cool, dry, and well-ventilated place.
In conclusion, p-bromoaniline is an important intermediate in the synthesis of a variety of chemicals. It is used as a starting material for the production of dyes, pharmaceuticals, and agrochemicals. However, it is a hazardous chemical and should be handled with caution due to its toxicity and flammability.