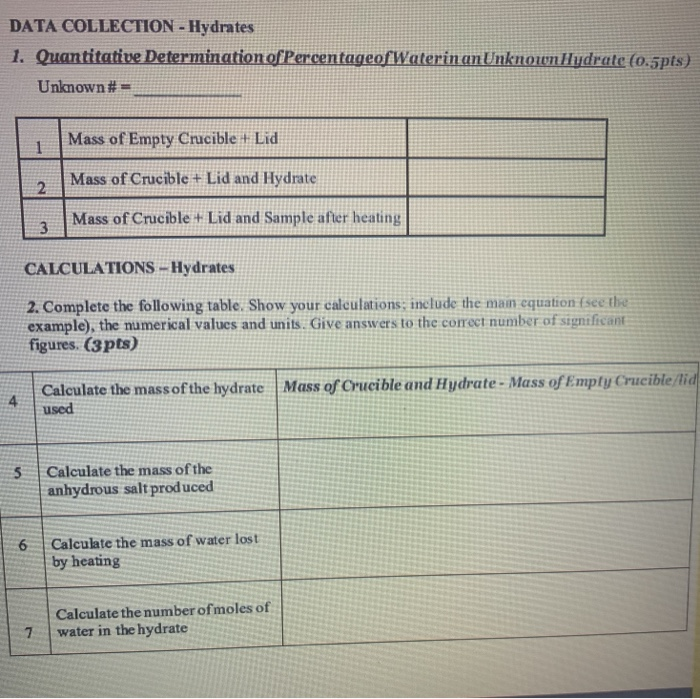
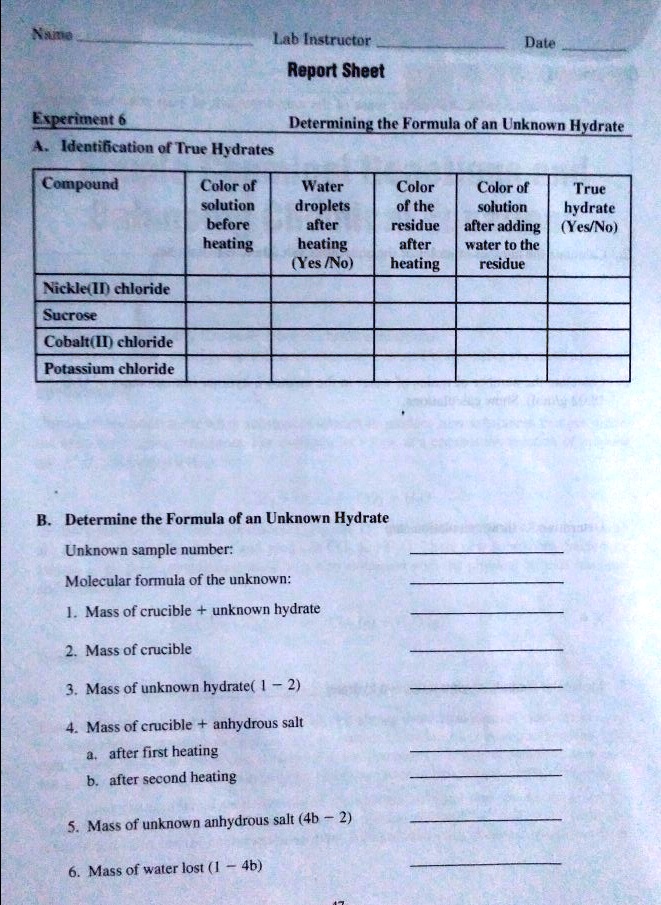
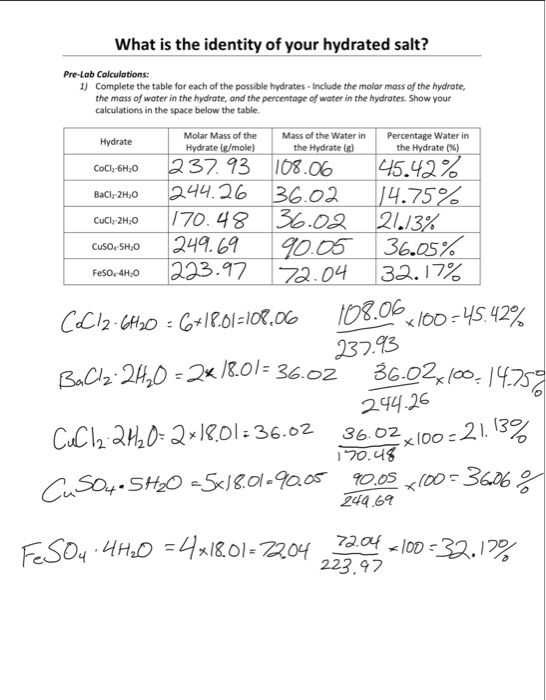
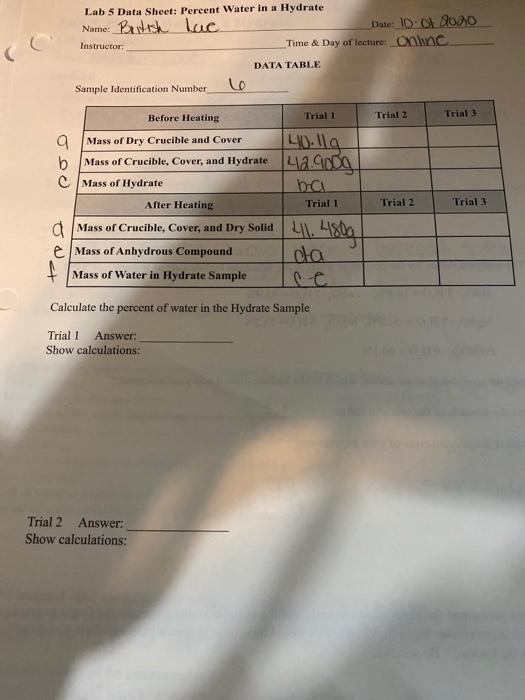
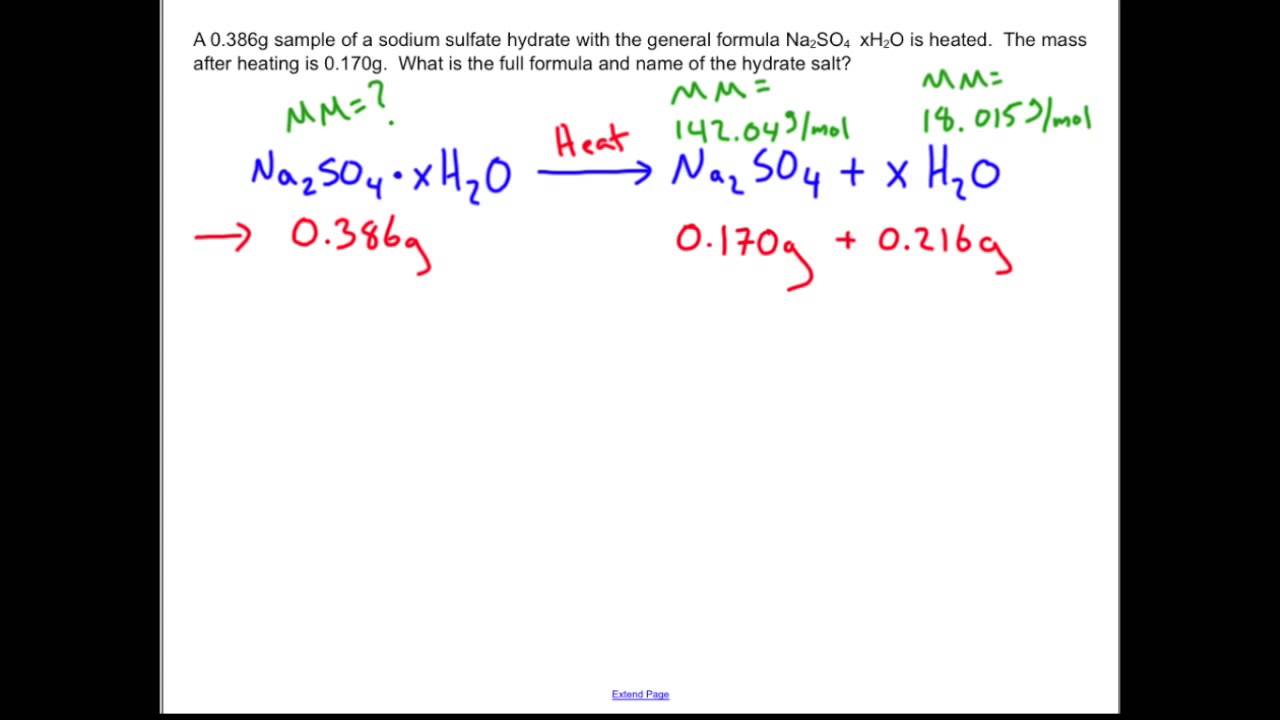
Identifying the percent water in a hydrate, or a compound that contains water molecules as part of its crystal structure, can be a useful way to understand its properties and potential uses. There are several methods that can be used to determine the percent water in an unknown hydrate, including weighing the sample before and after heating, using a calorimeter to measure the heat of hydration, and using spectroscopic techniques such as infrared (IR) spectroscopy.
One common method for identifying the percent water in a hydrate is to weigh the sample before and after heating. To do this, the sample is first weighed on a balance and then heated in a crucible until all of the water has been driven off. The crucible is then cooled and reweighed to determine the mass of the anhydrous (water-free) compound. The percent water in the hydrate can then be calculated using the following equation:
% water = (mass of water / mass of hydrate) x 100
Another method for determining the percent water in a hydrate is to use a calorimeter to measure the heat of hydration. This involves adding a known mass of the hydrate to a known mass of water and measuring the change in temperature. The heat of hydration is then calculated using the following equation:
q = (m1 x Cp1 x ΔT) + (m2 x Cp2 x ΔT)
where q is the heat of hydration, m1 is the mass of the hydrate, Cp1 is the specific heat of the hydrate, ΔT is the change in temperature, m2 is the mass of water, and Cp2 is the specific heat of water.
Finally, spectroscopic techniques such as IR spectroscopy can be used to identify the percent water in a hydrate. IR spectroscopy works by measuring the absorption of infrared light by a sample. Different functional groups absorb different wavelengths of infrared light, and by analyzing the absorption spectrum of a sample, it is possible to identify the functional groups present and therefore determine the composition of the compound.
In conclusion, there are several methods that can be used to determine the percent water in an unknown hydrate. Weighing the sample before and after heating, using a calorimeter to measure the heat of hydration, and using spectroscopic techniques such as IR spectroscopy are all effective ways to identify the percent water in a hydrate.