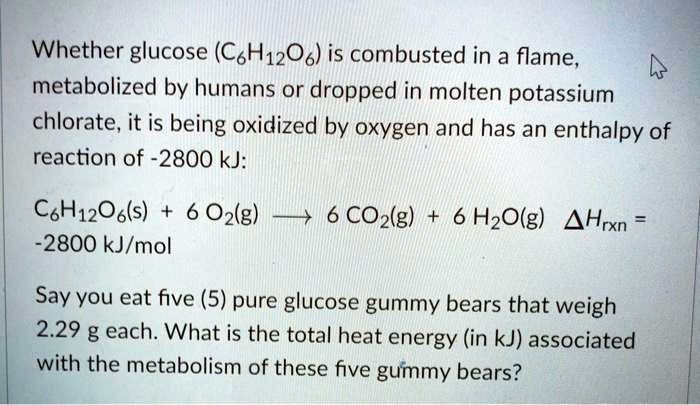Potassium chlorate is a chemical compound with the formula KClO3. It is a white crystalline solid that is highly soluble in water and is commonly used as an oxidizing agent in a variety of applications.
One well-known use of potassium chlorate is in the production of fireworks. When ignited, it produces a bright white light and a loud noise, making it an effective pyrotechnic ingredient. It is also used as a powerful oxidizing agent in the manufacturing of explosives, such as dynamite.
In addition to its use in pyrotechnics and explosives, potassium chlorate is also used in the production of matches and as a bleaching agent in the textile and paper industries. It is also used in some medical and laboratory settings as a source of oxygen.
Gummy bears, on the other hand, are a type of small, fruit-flavored gummy candy that are popular with children and adults alike. They are made from a mixture of sugar, corn syrup, and gelatin, and are often flavored with fruit juices or concentrates. Gummy bears are known for their soft, chewy texture and their bright, colorful appearance.
While potassium chlorate and gummy bears may seem like unrelated substances, they have actually been the subject of a popular science experiment involving the combination of the two. When a gummy bear is placed in a solution of potassium chlorate, the gummy bear will inflate and expand due to the release of gas from the chemical reaction between the two substances.
This experiment is often used to demonstrate the concept of chemical reactions and the release of gas. It is important to note, however, that the combination of potassium chlorate and gummy bears should not be ingested or handled in any way that could be potentially hazardous. Potassium chlorate is a toxic chemical and should be handled with caution.
In conclusion, potassium chlorate is a chemical compound with a variety of uses, including pyrotechnics and explosives, while gummy bears are a popular type of candy known for their soft, chewy texture and fruit flavors. While the combination of the two may make for an interesting science experiment, it is important to handle both substances with caution and not to ingest them.