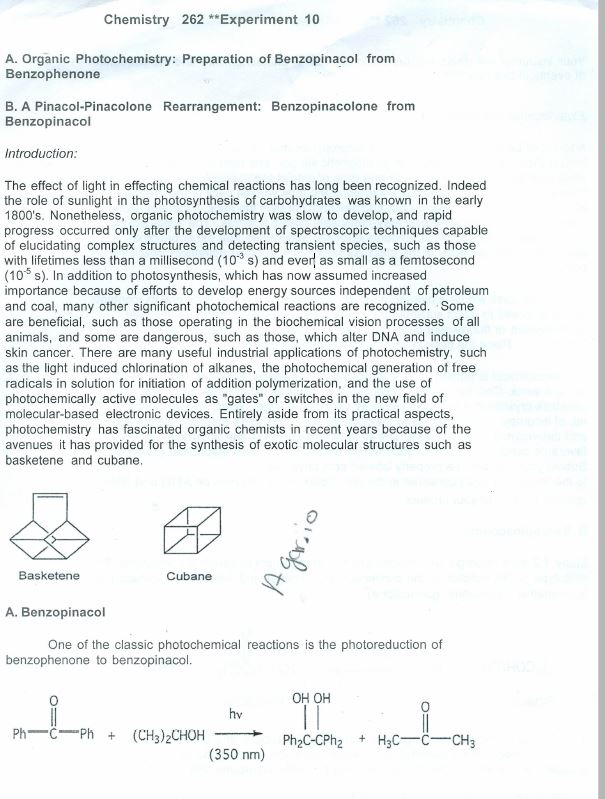
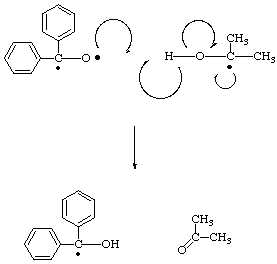
Benzopinacol, also known as benzopinacolone, is an organic compound that can be prepared from the starting material benzophenone through a series of chemical reactions. The synthesis of benzopinacol from benzophenone involves several steps, including the formation of a Grignard reagent, the conversion of the Grignard reagent to a ketone, and the reduction of the ketone to the final product. In this essay, we will outline the preparation of benzopinacol from benzophenone, including the necessary reagents, conditions, and mechanisms of each step.
The first step in the synthesis of benzopinacol is the formation of a Grignard reagent. Grignard reagents are a type of organometallic compound that are formed by the reaction of an alkyl or aryl halide with magnesium metal in an ether solvent. To prepare the Grignard reagent, benzophenone is dissolved in an ether solvent and cooled to a low temperature. Magnesium metal is then added to the solution, and the mixture is stirred until the magnesium has completely reacted with the benzophenone.
Once the Grignard reagent has been formed, it is converted to a ketone through the addition of a halogenated hydrocarbon, such as carbon tetrachloride or chloroform. The ketone is then reduced to the final product, benzopinacol, through the addition of a reducing agent, such as lithium aluminum hydride or sodium borohydride.
The mechanism of the Grignard reaction involves the formation of a complex between the magnesium and the halogen atom of the alkyl or aryl halide. The complex then undergoes a nucleophilic attack on the carbon atom of the halide, forming a carbon-magnesium bond and a halide ion. The resulting Grignard reagent is a nucleophile that can attack electrophilic carbon atoms, such as those found in aldehydes and ketones.
In the case of the synthesis of benzopinacol from benzophenone, the Grignard reagent attacks the electrophilic carbon atom of the benzophenone, forming a carbon-magnesium bond and a phenoxide ion. The phenoxide ion is then protonated by the halogenated hydrocarbon, forming a ketone. The ketone is then reduced to the final product, benzopinacol, through the addition of a reducing agent.
In conclusion, the synthesis of benzopinacol from benzophenone involves the formation of a Grignard reagent, the conversion of the Grignard reagent to a ketone, and the reduction of the ketone to the final product. This synthesis involves several steps and requires the use of various reagents and conditions, but it is a valuable method for the preparation of benzopinacol, which has a wide range of applications in organic chemistry.