Preparation of methyl 3 nitrobenzoate. CN105130820A 2023-01-06
Preparation of methyl 3 nitrobenzoate
Rating:
9,7/10
175
reviews
Methyl 3-nitrobenzoate is a chemical compound with the formula C8H7NO3. It is a white solid that is used as a precursor in the synthesis of various pharmaceuticals and dyes. The preparation of methyl 3-nitrobenzoate involves several steps that involve the use of hazardous chemicals and reactions that produce potentially harmful byproducts. Therefore, it is important to follow proper safety procedures and use appropriate protective equipment when preparing this compound.
The first step in the preparation of methyl 3-nitrobenzoate is the synthesis of nitrobenzene, which is a key intermediate in the synthesis of various nitro compounds. Nitrobenzene can be synthesized from benzene by nitration with a mixture of concentrated sulfuric acid and nitric acid. The nitration reaction is exothermic, and the temperature of the reaction mixture must be carefully controlled to prevent the formation of unwanted byproducts.
Once the nitrobenzene has been synthesized, it can be converted to methyl 3-nitrobenzoate by a series of reactions. The first step involves the reduction of the nitro group to an amine group using a reducing agent such as iron and hydrochloric acid. This reaction is followed by the conversion of the amine group to a methylamine group using formaldehyde and hydrochloric acid. Finally, the methylamine group is converted to a methyl group using hydrochloric acid and methanol.
It is important to note that all of the reactions involved in the synthesis of methyl 3-nitrobenzoate produce hazardous byproducts, including toxic gases and corrosive liquids. Therefore, it is essential to follow proper safety procedures and use appropriate protective equipment, such as goggles, gloves, and a lab coat, when preparing this compound.
In summary, the preparation of methyl 3-nitrobenzoate involves a series of reactions that involve the use of hazardous chemicals and produce potentially harmful byproducts. Therefore, it is important to follow proper safety procedures and use appropriate protective equipment when preparing this compound.
Synthesis of Methyl 3
Teaching notes The nitration of methyl benzoate is an example of electrophilic substitution. Google has not performed a legal analysis and makes no representation as to the accuracy of the date listed. However the 3-position is less deactivated towards nitration than the other positions owing to the relative stability of the different intermediates. Example 3: be with to 1000ml in the four-hole bottle of reflux exchanger, water trap and drop into 200g3-nitro o-Xylol, 200g orthodichlorobenzene, 200g n-caproic acid, 10g Cobaltous diacetate, 14g manganese acetate, 16g tetrabromoethane, pass into oxygen and keep 1. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed. Allow this mixture to cool. ABSTRACT In this experiment chemical technics are used to prepare methyl 3- nitrobenzoate.
Next
Preparation of Methyl 3

NOTE: The nitrating mixture is very corrosive. CN105130820A - New 2-methyl-3-nitrobenzoic acid preparation method - Google Patents CN105130820A - New 2-methyl-3-nitrobenzoic acid preparation method - Google Patents New 2-methyl-3-nitrobenzoic acid preparation method Info Publication number CN105130820A CN105130820A CN201510492435. INTRODUCTION Higher density of benzene rings is the one of the things that makes aromatic substitution to be electrophilic. These two solutions are then being added to ice water in the same beaker and mixed together until white granules are formed. Place a test tube filled with DI water and a test tube filled with methanol in an ice bath. Purification and analysis Methyl 3-nitrobenzoate is insoluble in water but soluble in hot ethanol. This is the nitrating mixture.
Next
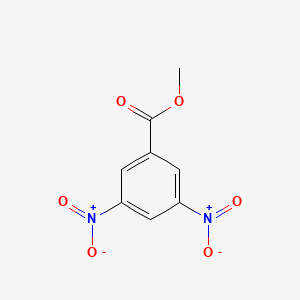
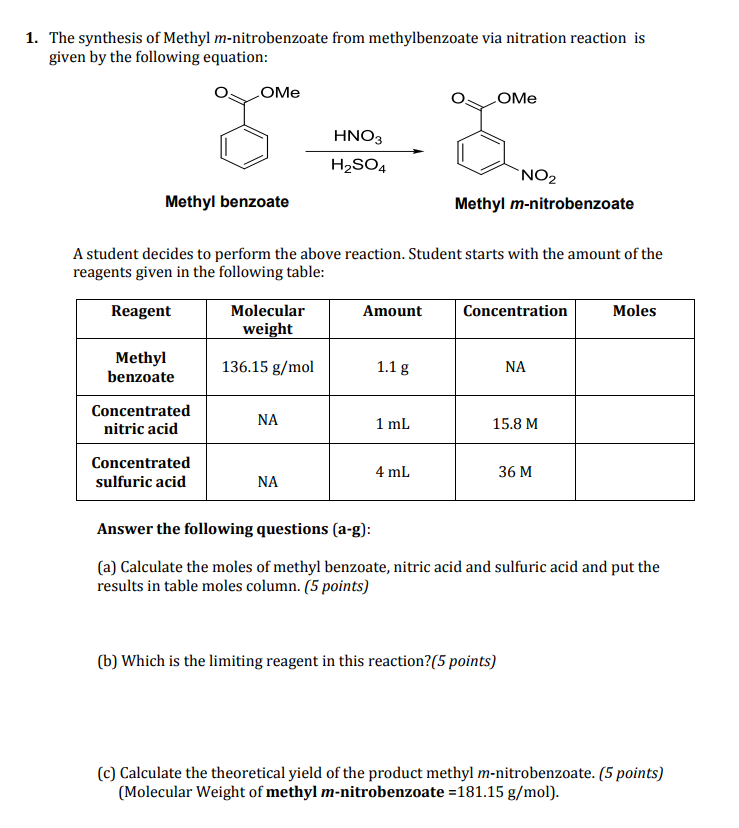
Nitration of methyl benzoate

The carbonyl group withdraws electron density from the ring deactivating it towards electrophilic substitution. The first thing to be done here is to mix methyl benzene with nitric acid and that is a hazard substance so a precaution should be paid at this stage. Therefore the reaction is regioselective for nitration at the 3-position. The species reacting with the aromatic ring is usually a positive ion or the end of a dipole. Then the nitric acid is also being added with the sulfuric acid.
Next
preparation of methyl 3

Stir the reaction mixture as the addition is made. In another test tube TT1 add 0. Nitration is one of the most important examples of electrophilic substitution. Claims 5 The preparation method that 1. The electrophile in nitration is the nitronium ion which is generated from nitric acid by protonation and loss of water , using sulphuric acid as the dehydrating agent.
Next
CN105130820A
According to the present invention, the oxygen in the air is used to replace nitric acid to oxidize the 3-nitro-o-xylene into the 2-methyl-3-nitrobenzoic acid, the oxygen is used to oxidize the 3-nitro-o-xylene, the reaction is the normal pressure reaction, the mother liquor is applied, the yield is up to 80%, and the method is the clean production method with characteristics of low risk, low pollution and low raw material cost. The lab should produce skills that allow for the characterization of the formation of the electrophile and the meta- product through the nitration electrophilic aromatic substitution reaction, the characterization of the reaction mechanism, the differentiation of the products and byproducts, and data compilation and analysis. IDA FLAMMABLE — highly flammable liquid and vapour; MODERATE HAZARD — harmful if swallowed, may cause damage to organs can be used instead. The solution is then filtrated to get methyl 3-nitrobenzoate. Rings of benzene are the ones components that are found in many natural product, it is also found other important product. Existing 2-methyl-3-nitro phenylformic acid product is oxidized through dust technology by 3-nitro o-Xylol, and autoclave must be used to carry out reaction under high pressure, dangerous large, explosive, and larger containing the contaminated wastewater of nitric acid, by product 2-methyl-6-nitrobenzoic acid, 3-nitrophthalic acid is more, and yield can only accomplish 27%, because this method is dangerous large, pollute large, cause enterprise normally to produce. .
Next
Preparation of methyl
Object of the present invention is in order to overcome above-mentioned prior art Problems existing, and the preparation method that a kind of 2-methyl-3-nitro phenylformic acid is new is provided, oxygen in the inventive method air substitutes nitric acid oxidation 3-nitro o-Xylol and becomes 2-methyl-3-nitro phenylformic acid, and adopt dioxygen oxidation 3-nitro o-Xylol, synthesis under normal pressure, mother liquid recycle, yield is up to 80%, the present invention be dangerous little, pollute little, that material cost is low clean preparation method. Procedure Preparation of methyl 3-nitrobenzoate a Weigh 2. Granted Application number CN201510492435. The recrystallization is carried out from a mixture of water and ethanol. Cool this mixture by partially immersing the flask in an ice-water bath.
Next
Splash proof goggles or a face shield should be worn together with chemical resistant gloves. Example 1: be with to 1000ml in the four-hole bottle of reflux exchanger, water trap and drop into 200g3-nitro o-Xylol, 700g orthodichlorobenzene, 200g n-caproic acid, 12g Cobaltous diacetate, 12g manganese acetate, 6g tetrabromoethane, pass into oxygen and keep 1. The preparation method that 2-methyl-3-nitro phenylformic acid is new, it is characterized in that carrying out according to the following steps: add 3-nitro o-Xylol, organic solvent and catalyzer in the reactor, pass into dioxygen oxidation, oxidizing temperature is at 90-100 DEG C, when the mass concentration of 3-nitro o-Xylol is less than 1% for reaction end in reactor, cold filtration obtains crude product, disposing mother liquor recycle; Crude product obtains 2-methyl-3-nitro phenylformic acid finished product through conventional alkalization method, activated carbon decolorizing, souring method successively. Stir the crushed ice throughout. HUANGSHI LIFUDA MEDICINE CHEMICAL Co Ltd Original Assignee HUANGSHI LIFUDA MEDICINE CHEMICAL Co Ltd Priority date The priority date is an assumption and is not a legal conclusion. After recrystallization was done the appropriate solvent was used to run TLC plate readings.
Next

Example 2: be with to 1000ml in the four-hole bottle of reflux exchanger, water trap and drop into 200g3-nitro o-Xylol, 700g orthodichlorobenzene, 300g n-caproic acid, 4g Cobaltous diacetate, 2g manganese acetate, 4g tetrabromoethane, pass into air and keep 3. The reaction is shown below : HNO 3 + 2H 2 SO 4. Lesson organisation To synthesise and recrystallize a sample of methyl 3-nitrobenzoate will take about 1½ h. During the addition keep the temperature of the reaction mixture below 6 °C. Apparatus Chemicals conical flasks 1 × 50 cm 3 and 1 × 100 cm 3 balance spatula 10 cm 3 measuring cylinders × 2 glass dropping pipette ice-water bath glass rod test tube thermometer 0—100 ° C Buchner funnel apparatus melting point apparatus methyl benzoate MODERATE HAZARD — harmful if swallowed concentrated sulfuric acid CORROSIVE — causes severe skin burns and eye damage concentrated nitric acid CORROSIVE AND OXIDISING — causes severe skin burns and eye damage.
Next

TITLE The experiment where chemistry technics are used so that methyl 3- nitrobenzoete is prepared. Google has not performed a legal analysis and makes no representation or warranty as to the accuracy of the list. May intensify fire methyl 3-nitrobenzoate no known hazards ethanol FLAMMABLE — highly flammable liquid and vapour. The reaction is regioselective and produces predominantly methyl 3-nitrobenzoate. Swirl and cool the mixture in the ice bath.
Next
The nitrating mixture must be made in situ as required and kept cool throughout. Solid methyl 3-nitrobenzoate will form. Description 2-methyl-3-nitro phenylformic acid product is through reduction reaction, diazotization reaction, methoxylation makes 2-methyl-3-methoxybenzoic acid, and 2-methyl-3-methoxybenzoic acid is the important intermediate preparing new and effective low toxicity bishydrazide sterilant methoxyfenozide, very in short supply on Vehicles Collected from Market. In this experiment the students nitrate methyl benzoate. The product is then recrystallised using methanol and obtain the milting point of both products.
Next








