In this lab report, we will discuss the process of separating salt and sand through the use of a physical separation technique. The purpose of this experiment is to demonstrate the effectiveness of this method in separating two substances with different physical properties.
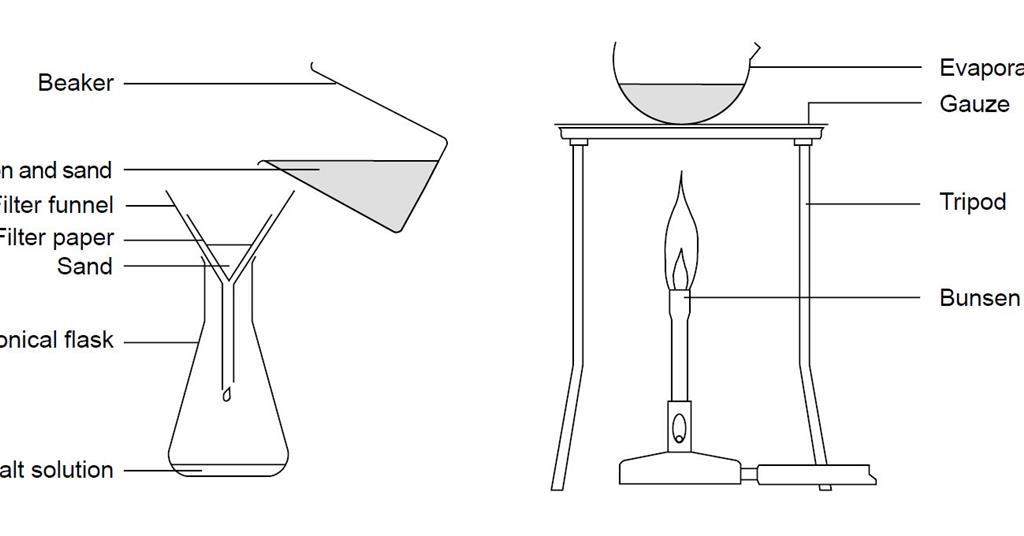
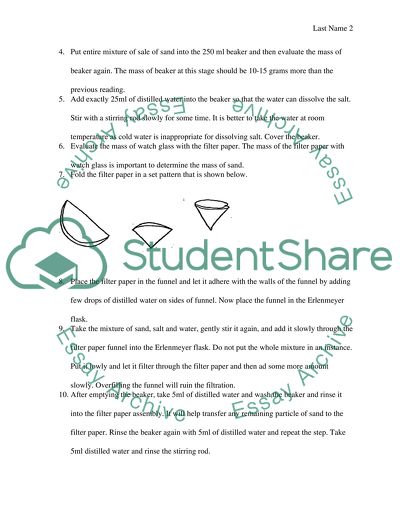
The materials needed for this experiment include a beaker, water, salt, sand, and a filter paper. To begin the experiment, we first measured out equal amounts of salt and sand and placed them in the beaker. Next, we added water to the beaker and stirred the mixture until the salt dissolved in the water.
Once the salt had dissolved, we used the filter paper to filter out the solid particles of sand. The filter paper was placed in a funnel and placed over a clean, empty beaker. The saltwater solution was then poured through the filter paper, allowing the liquid to pass through while trapping the solid sand particles.
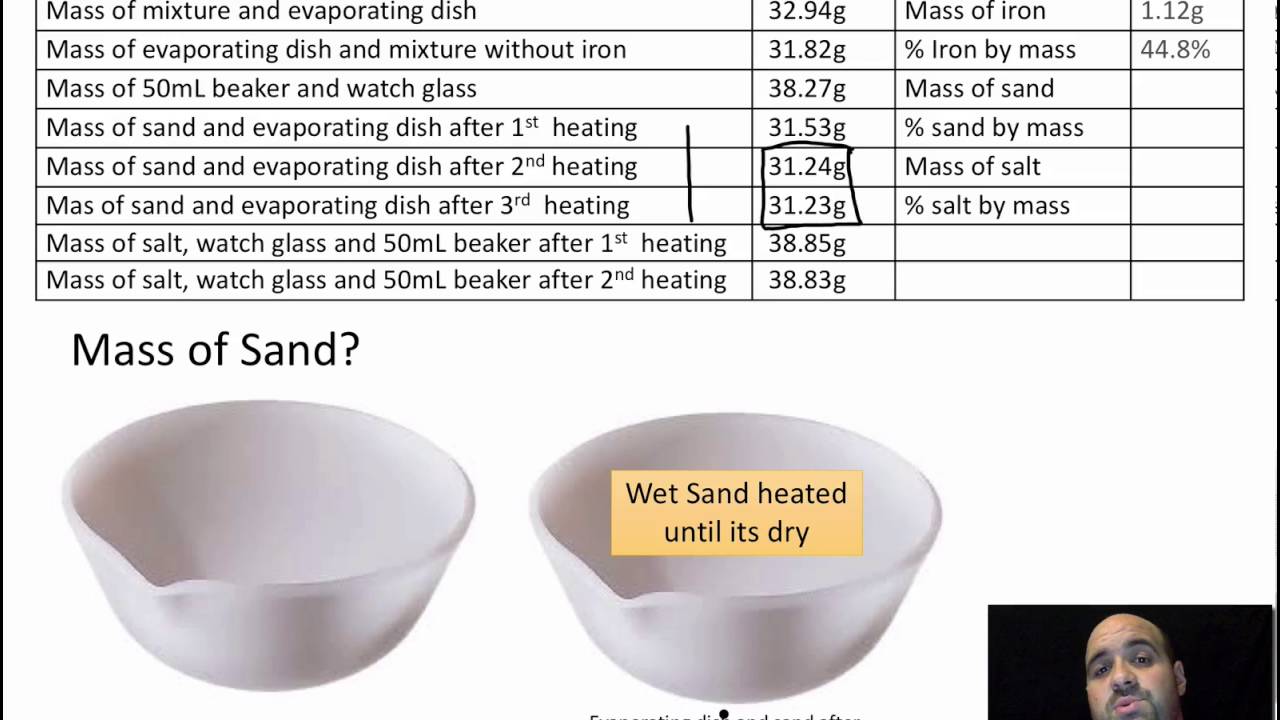
After all of the saltwater solution had been filtered, we were left with a beaker containing the sand and a beaker containing the saltwater solution. To separate the salt from the water, we allowed the saltwater solution to evaporate, leaving behind the salt crystals.
The results of this experiment showed that it is possible to effectively separate salt and sand through the use of a physical separation technique. By using the filter paper to separate the solid sand particles from the liquid saltwater solution, we were able to successfully isolate the two substances.
Overall, this experiment was a success and demonstrated the effectiveness of physical separation techniques in separating substances with different physical properties. This knowledge can be useful in a variety of real-world applications, such as separating contaminants from water or extracting minerals from ore.