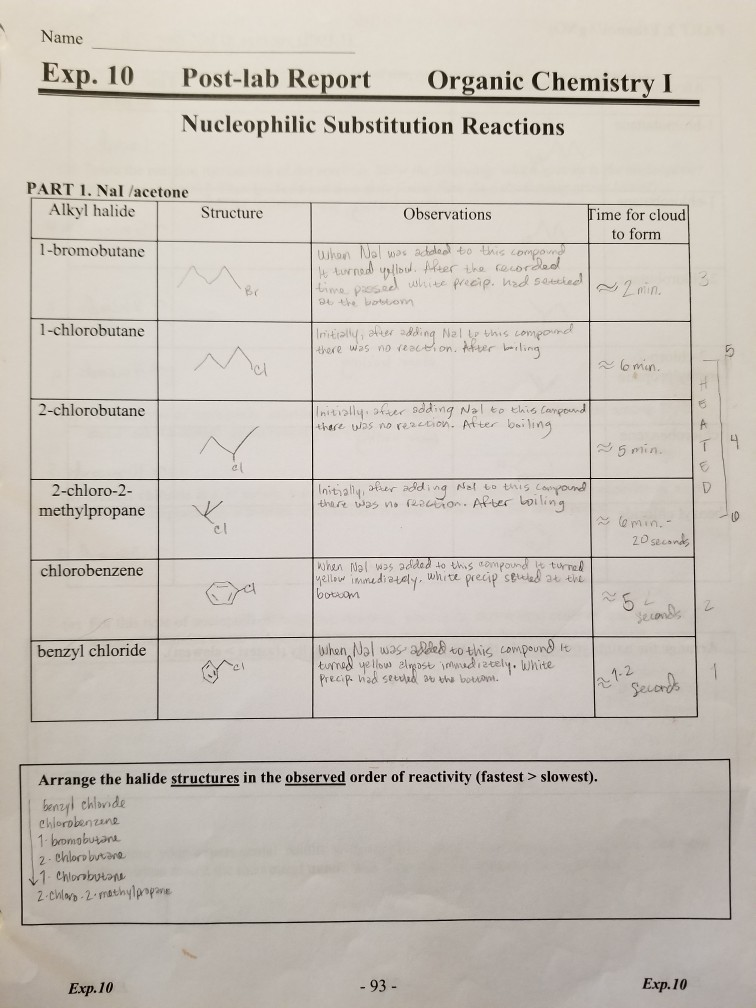
In an sn1 or sn2 reaction of alkyl halides, a halogen atom is replaced with a nucleophile. These reactions are important in organic chemistry because they allow for the synthesis of a wide range of compounds.
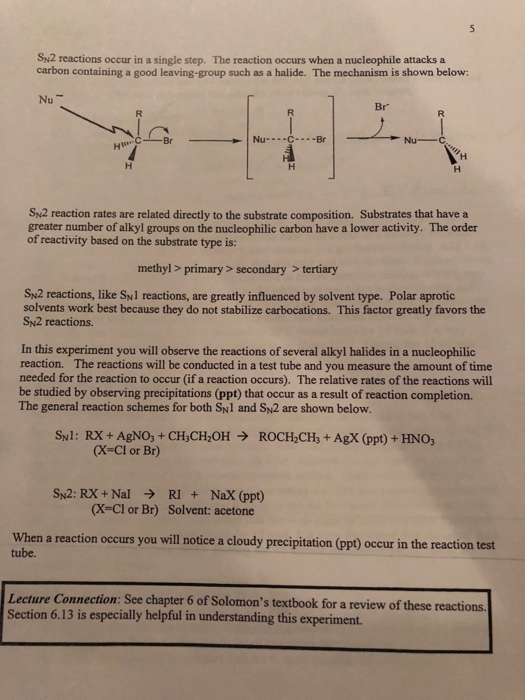
In an sn1 reaction, the substitution occurs in a two-step process. First, the alkyl halide undergoes a unimolecular substitution, where the halogen atom is replaced by a nucleophile. This step is rate-determining, meaning that it determines the overall rate of the reaction. The second step is the formation of the product, which occurs rapidly.
One factor that affects the rate of an sn1 reaction is the stability of the carbocation intermediate that is formed during the unimolecular substitution. The more stable the carbocation, the faster the sn1 reaction will occur. This is because the carbocation intermediate is more likely to be formed if it is stable, and a stable intermediate will also be more likely to react with the nucleophile to form the product.
In contrast, an sn2 reaction occurs in a single step, where the nucleophile attacks the carbon atom bonded to the halogen atom and displaces it. The rate of an sn2 reaction is dependent on the concentration of the nucleophile and the substrate, as well as the nature of the nucleophile and substrate.
One factor that affects the rate of an sn2 reaction is the nucleophile's ability to stabilize the intermediate formed during the reaction. A nucleophile with a high electron density will be more likely to stabilize the intermediate, leading to a faster sn2 reaction.
The choice of solvent can also influence the rate of an sn1 or sn2 reaction. Polar solvents, such as water, favor sn2 reactions, while nonpolar solvents, such as hexane, favor sn1 reactions.
In the laboratory, alkyl halides can be synthesized using a variety of methods, including the halogenation of alkanes and the substitution of haloalkanes with nucleophiles. These reactions can be carried out using a variety of reagents, including halogen gas, halogen acids, and haloalkanes.
Overall, sn1 and sn2 reactions of alkyl halides are important tools in organic chemistry, allowing for the synthesis of a wide range of compounds. Understanding the factors that affect the rate and mechanism of these reactions is essential for the successful design and execution of these reactions in the laboratory.