Specific weight, also known as specific gravity, is a measure of the density of a substance relative to the density of water. It is defined as the ratio of the density of the substance to the density of water at a specific temperature. The specific weight of a substance is an important physical property that can be used to identify and characterize different materials.
In the case of alcohol, the specific weight will depend on the type of alcohol and the concentration of the solution. Alcohols are organic compounds that contain a hydroxyl (-OH) group bonded to a carbon atom. There are several types of alcohols, including methanol, ethanol, and propanol, each of which has a different molecular structure and therefore a different specific weight.
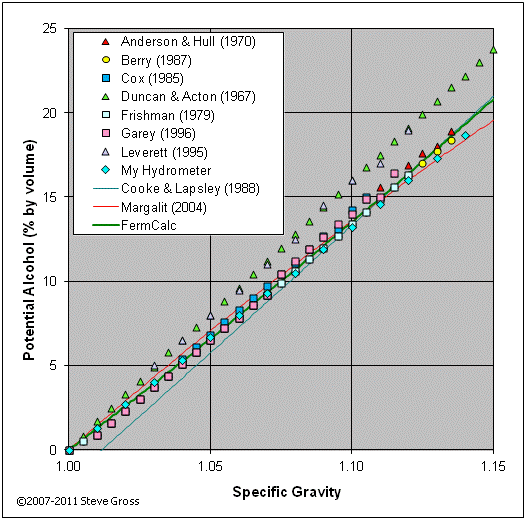
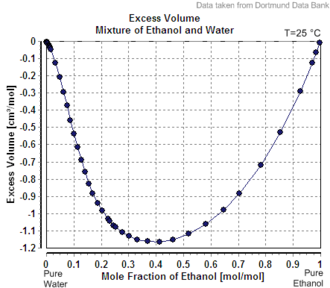
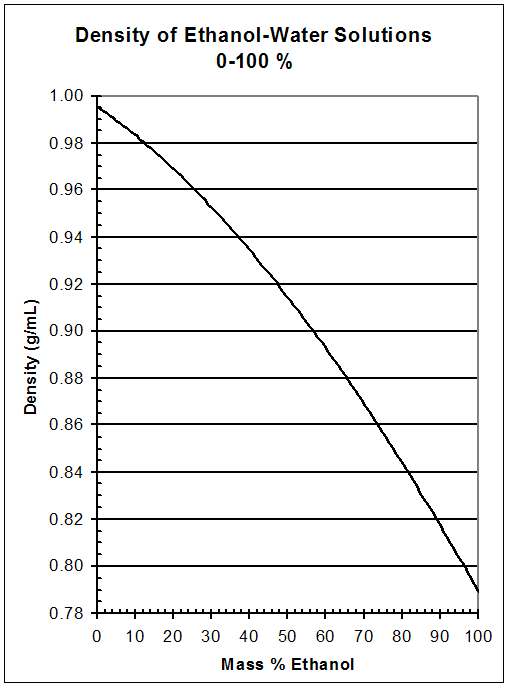
For example, the specific weight of ethanol, which is the active ingredient in alcoholic beverages, is 0.789 g/cm^3 at 20 degrees Celsius. This means that 1 cm^3 of ethanol will weigh 0.789 grams. The specific weight of ethanol can vary slightly depending on the concentration of the solution. For instance, the specific weight of a solution that is 50% ethanol and 50% water will be lower than the specific weight of a solution that is 95% ethanol and 5% water.
The specific weight of alcohol can be important in various applications. In the field of chemical engineering, for instance, the specific weight of a substance can be used to calculate the mass flow rate of a fluid through a pipe or other type of conduit. It can also be used in the design of tanks and other containers, as the specific weight will affect the buoyancy of the substance.
In conclusion, the specific weight of alcohol is a measure of the density of the substance relative to the density of water. It is an important physical property that can be used to identify and characterize different types of alcohols, as well as to calculate the mass flow rate of fluids and design containers. The specific weight of alcohol can vary depending on the type of alcohol and the concentration of the solution.