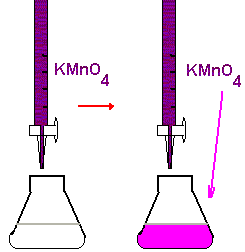
Potassium permanganate, also known as KMnO4, is a chemical compound that is widely used in a variety of applications, including water treatment, disinfection, and bleaching. It is an effective oxidizing agent and can be used to remove contaminants from water, kill bacteria and other microorganisms, and remove stains from fabrics and surfaces.
One important aspect of using potassium permanganate is standardization, which refers to the process of ensuring that the chemical is of a consistent strength and purity. This is necessary in order to accurately measure and control the concentration of potassium permanganate in various solutions and to ensure that it is effective in achieving the desired results.
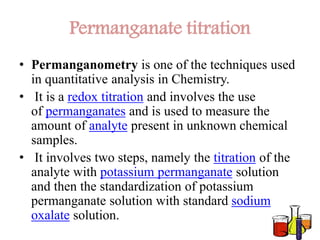
There are several methods for standardizing potassium permanganate, each of which has its own advantages and limitations. One common method is to use a standardized solution of potassium permanganate, which is prepared by dissolving a known amount of the chemical in water and measuring the concentration with a suitable instrument, such as a spectrophotometer.
Another method for standardizing potassium permanganate is to use a primary reference standard, such as potassium dichromate or potassium chloride, which has a known concentration and can be used to titrate the potassium permanganate solution. This involves adding a measured amount of the primary reference standard to the potassium permanganate solution until the reaction is complete, and then measuring the concentration of the resulting solution.
Standardization of potassium permanganate is important in order to ensure the accuracy and reliability of test results and to ensure that the chemical is used safely and effectively. It is also important to regularly check the expiration date of potassium permanganate and to discard it if it has expired, as the strength of the chemical may degrade over time.
In conclusion, standardization of potassium permanganate is a crucial step in ensuring the accuracy and effectiveness of this chemical compound. By regularly checking the strength and purity of potassium permanganate solutions, users can ensure that they are using the chemical safely and effectively, and can achieve consistent and reliable results.