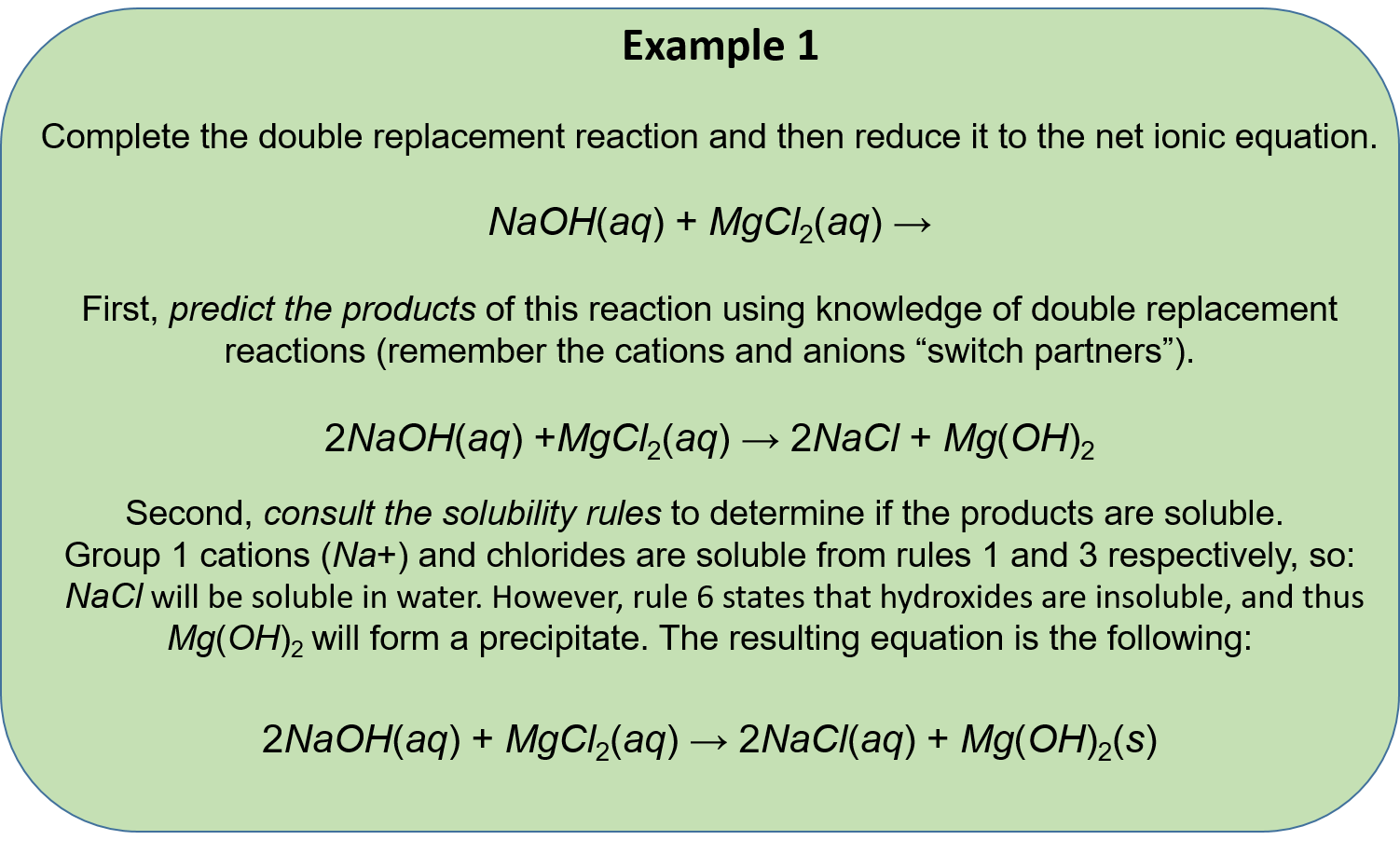
Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. It is an important concept in chemistry that helps us to understand how much of a reactant is needed to produce a given amount of product. In precipitation reactions, stoichiometry plays a crucial role in determining the amount of reactants that are needed to produce a precipitate, which is a solid that forms and separates out of a solution during a chemical reaction.
Precipitation reactions occur when two aqueous solutions are mixed together and a solid precipitate forms as a result of the reaction. The reactants in these reactions are usually ions, which are charged particles that are found in solution. The ions can either be cations, which are positively charged, or anions, which are negatively charged. In order to form a precipitate, the cations and anions must combine to form an ionic compound, which is a solid with a crystal structure.
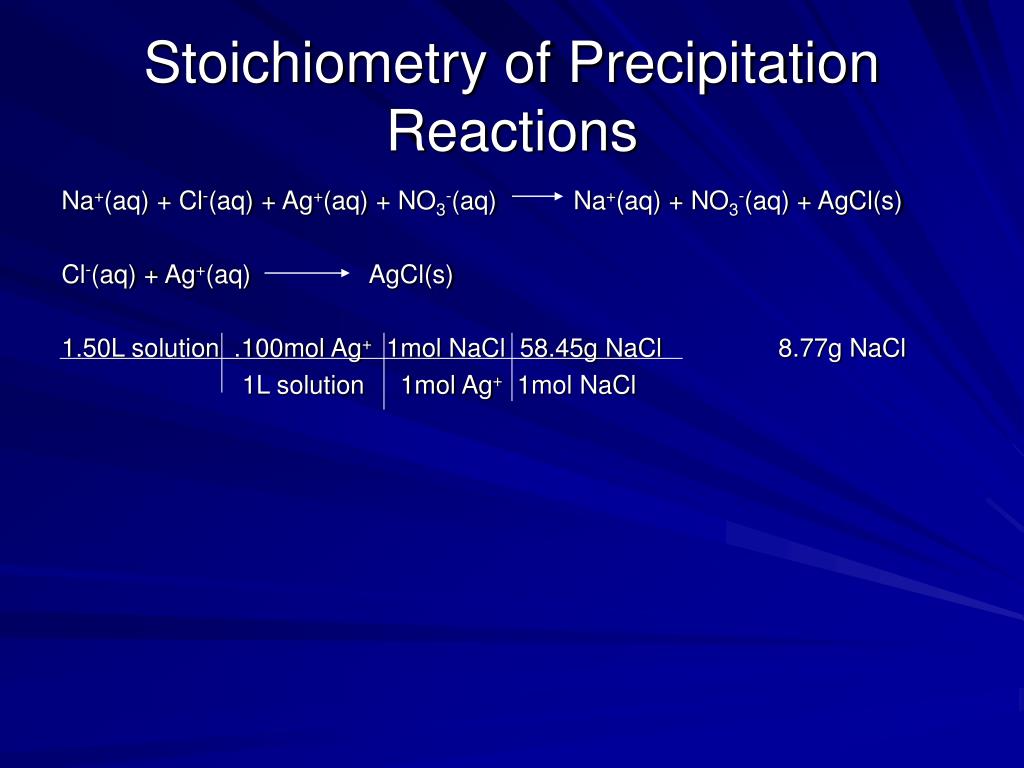
The stoichiometry of a precipitation reaction is determined by the balanced chemical equation for the reaction. The balanced chemical equation shows the reactants and products of the reaction, as well as their relative proportions. For example, the balanced chemical equation for the precipitation of barium sulfate from a solution of barium chloride and sodium sulfate is:
BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2 NaCl (aq)
This equation shows that for every one mole of barium chloride that reacts with one mole of sodium sulfate, one mole of barium sulfate is produced and two moles of sodium chloride are also produced.
To determine the amount of reactants needed to produce a certain amount of precipitate, we can use the concept of stoichiometric ratios. The stoichiometric ratio is the ratio of the moles of one reactant to the moles of another reactant in a chemical equation. For example, in the above equation, the stoichiometric ratio of barium chloride to sodium sulfate is 1:1, meaning that for every one mole of barium chloride that is needed, one mole of sodium sulfate is also needed.
To calculate the amount of reactants needed to produce a given amount of precipitate, we can use the following steps:
- Write the balanced chemical equation for the precipitation reaction.
- Determine the molar mass of the precipitate. The molar mass is the mass of one mole of the substance, and it is typically expressed in grams per mole (g/mol).
- Calculate the number of moles of precipitate that are needed. To do this, divide the desired mass of the precipitate by its molar mass.
- Determine the stoichiometric ratio of the reactants. This can be done by looking at the coefficients in the balanced chemical equation.
- Calculate the number of moles of each reactant needed. To do this, divide the number of moles of precipitate needed by the stoichiometric ratio of the reactants.
For example, suppose we want to produce 50 grams of barium sulfate by reacting barium chloride with sodium sulfate. Using the above steps, we can calculate the amount of reactants needed as follows:
- The balanced chemical equation for the reaction is: BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2 NaCl (aq)
- The molar mass of barium sulfate is 233.4 g/mol.
- The number of moles of barium sulfate needed is 50 g / 233.4 g/