Sulphuric acid (H2SO4) and sodium hydroxide (NaOH) are two highly corrosive and caustic chemicals that are widely used in a variety of industrial and laboratory applications. Both of these chemicals are strong electrolytes and can be classified as acids and bases, respectively, based on their ability to donate or accept protons.
Sulphuric acid is a strong acid that is commonly used in the production of fertilizers, detergents, and dyes. It is also used in the refining of petroleum and the production of pharmaceuticals and plastics. The chemical formula for sulphuric acid is H2SO4, and it is a clear, colorless, and oily liquid that is highly soluble in water. When dissolved in water, sulphuric acid ionizes completely to form hydrogen ions (H+) and sulphate ions (SO42-).
On the other hand, sodium hydroxide is a strong base that is commonly used in the manufacture of soap, detergents, and paper. It is also used in the production of textiles, dyes, and pharmaceuticals. The chemical formula for sodium hydroxide is NaOH, and it is a white, crystal-like solid that is highly soluble in water. When dissolved in water, sodium hydroxide ionizes completely to form hydroxide ions (OH-) and sodium ions (Na+).
When sulphuric acid and sodium hydroxide are mixed together, they react to form salt and water. This reaction is known as a neutralization reaction, and it is an exothermic reaction, meaning that it releases heat. The balanced chemical equation for the reaction between sulphuric acid and sodium hydroxide is as follows:
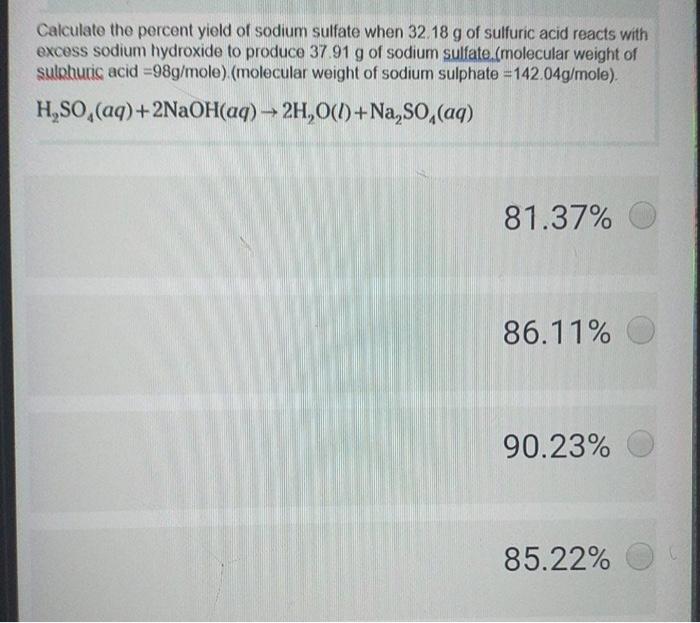
H2SO4 + 2NaOH -> Na2SO4 + 2H2O
The reaction between sulphuric acid and sodium hydroxide is a highly exothermic reaction, and it is important to handle these chemicals with caution. Both sulphuric acid and sodium hydroxide are corrosive and can cause burns to the skin and eyes. In addition, the vapors produced by these chemicals can be harmful if inhaled. It is important to wear appropriate protective gear, such as goggles and gloves, when handling these chemicals.
In conclusion, sulphuric acid and sodium hydroxide are two highly corrosive and caustic chemicals that are widely used in a variety of industrial and laboratory applications. They are both strong electrolytes and can be classified as acids and bases, respectively. When mixed together, they react to form salt and water in a neutralization reaction. It is important to handle these chemicals with caution due to their corrosive nature and the potential for harmful vapors.