"My Life had stood - a Loaded Gun" is a poem written by Emily Dickinson that explores themes of femininity, power, and the complexities of identity. Through the metaphor of a loaded gun, Dickinson delves into the idea that women are often expected to conform to societal expectations and roles, and that they may feel trapped and silenced by these expectations.
At the same as the speaker in the poem, the loaded gun represents the potential for power and agency, but also the burden and danger that comes with it. The gun is "loaded" with the expectations and roles that society has placed on the speaker, and she is constantly "cocked" and "ready" to perform and fulfill these expectations. The speaker is aware of the power she holds, but also recognizes that she is at the mercy of those who would "finger" and "handle" her, suggesting that she does not have complete control over her own body or identity.
The poem also touches on the theme of femininity, as the speaker is described as being "tender" and "gentle," traits that are often associated with traditional ideas of femininity. However, the speaker also asserts her strength and power, stating that she is "deadly," and that she "could" and "would" act if necessary. This tension between traditional femininity and the power and agency that comes with it is a common theme in feminist literature, and it highlights the complexities and contradictions that many women face in their lives.
In terms of a feminist analysis, "My Life had stood - a Loaded Gun" can be seen as a commentary on the ways in which society tries to control and define women's roles and identities. The metaphor of the loaded gun suggests that women are expected to be ready and willing to fulfill the expectations placed upon them, but that they may also feel trapped and silenced by these expectations. The poem also highlights the power and agency that women have, even if it is often suppressed or ignored by those around them. Overall, "My Life had stood - a Loaded Gun" is a powerful and thought-provoking poem that explores themes of femininity, power, and identity in a unique and compelling way.
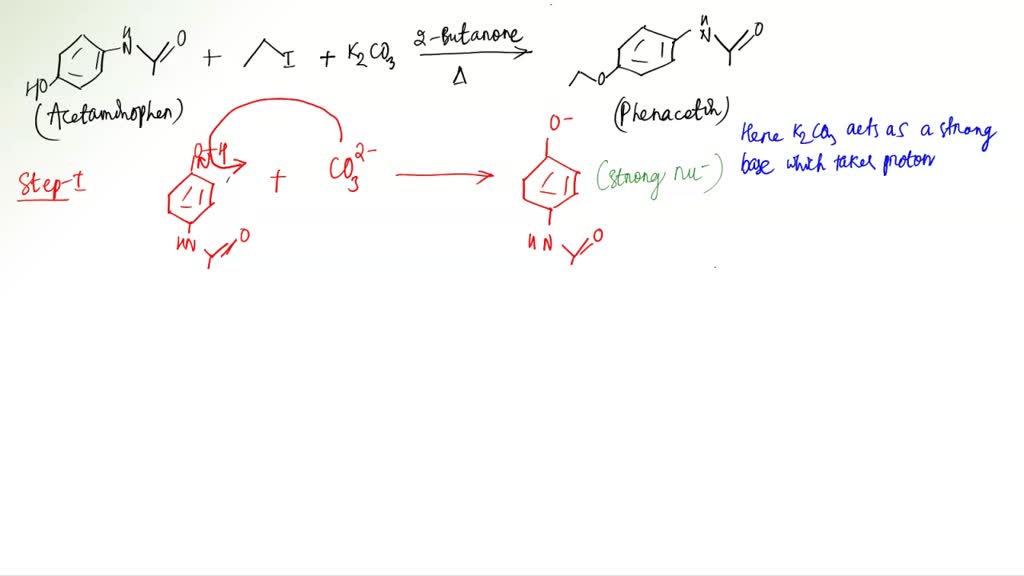
Williamson Ether Synthesis: Preparation of Phenacetin from Acetaminophen Flashcards

Tylenol is one of the most common OTC drugs available around the world. After the time period, the flask was cooled in an ice-water bath and 3 M HCl was added dropwise with swirling. Slightly less than half of product was lost from 0. Percent Yields and Theoretical Yields Table 5: Summary of Reaction Table Compound Molecular Weight Rxn Weight or Volume g or mL 1 g mmol Equivalents 151 D or M Acetaminophe n Potassium Carbonate 8 1 138 2 g 18 2 Iodoethane 155 72 1 0 3 1 mL 15 mL 12 167 1 19 Theoretical yield the theoretical yield is given : Theoretical Yield Starting Volume x Density x Molecular Weight x Reaction Stoichiometry x Molecular Weight 1 g x 1 mol 151 x 1 mol Acetaminophen 1 mol Phenacetin x 179 g 1 mol 1 grams of phenacetin Theoretical yield Percent yield actual yield theoretical yield x 100 Actual yield. We mended this joining another laboratory group and conducting the rest of the experiment with their materials. It can be due to the conditions of the leaving group, temperature and solvent. Source: Carberry and Carreon, 2012 In this experiment p-Aminophenol, also known as Tylenol, was constructed from treating p-Aminophenol with acetic anhydride.
Lab blog.sigma-systems.com

. Since it is an Sn2 reaction, the methyl and primary alkyl halides react more quickly than the secondary or tertiary alkyl halides, react as quickly. Experimental Procedure Obtain and crush 4 tablets of 325mg strength Tylenol using a mortar and pestle, this should add up to 1 acetaminophen. Followed by 1 mL of methanol through the top of the alumina. The cloudy solution should become clear as the is added. These crystals were then dried, weighed, and melting point was determined.
Synthesis of Phenacetin From Acetaminophen blog.sigma-systems.com

The hydrogen that is phenolic then proceeds to deprotonate due to the fact that it is acidic hydrogen. The melting point is observed via a magnifying lens and temperature is recorded as soon as the solid begins to melt. . Likewise, phenacetin used to 2 Shrestha be one of the most commonly used analgesic and antipyretic NSAID drugs. Some possible errors that might have occurred in the experiment might have been during recrystallization. The mechanism of the reaction is as follows: In this lab, a conical vial was first prepared containing methyl ethyl ketone MEK , acetaminophen, K 2 CO 3 , ethyl iodide, and a spin vane.