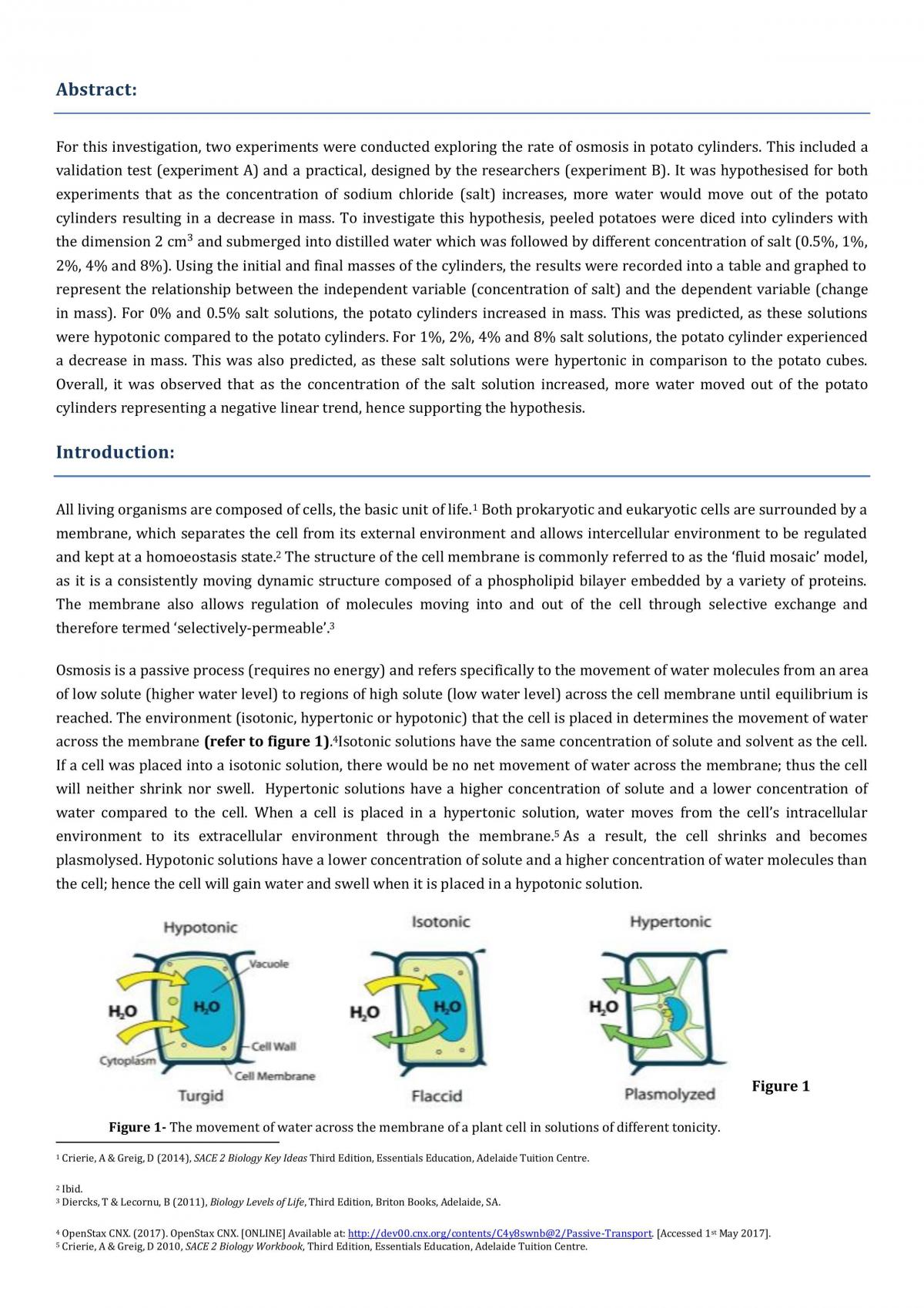
Osmosis is the movement of water molecules through a semipermeable membrane from an area of high water concentration to an area of low water concentration. In the context of potato cells, osmosis occurs when the potato cells are placed in a solution with a different salt concentration than the cells themselves.
When the salt concentration in the solution is higher than the concentration inside the potato cells, the water inside the cells will start to move out of the cells and into the solution. This is because the water molecules are attracted to the salt ions in the solution, which creates a higher concentration of water outside the cells than inside. As a result, the potato cells will lose water and become dehydrated, causing them to shrink.
On the other hand, if the salt concentration in the solution is lower than the concentration inside the potato cells, the water inside the cells will start to move into the solution. This is because the water molecules are attracted to the higher concentration of water in the solution, which creates a lower concentration of water inside the cells. As a result, the potato cells will gain water and become swollen, causing them to increase in size.
The effect of salt concentration on osmosis in potato cells can be observed through a simple experiment. To do this, you will need several potato slices, different salt solutions of varying concentrations, and a balance to measure the weight of the potato slices. First, you will need to weigh the potato slices and record their initial weight. Then, you will need to place each potato slice in a separate container with a different salt solution and let them sit for a few hours. After this time, you will need to remove the potato slices from the solutions and weigh them again.
If you compare the initial weight of the potato slices to the final weight, you will see that the weight of the potato slices placed in a solution with a higher salt concentration will have decreased, while the weight of the potato slices placed in a solution with a lower salt concentration will have increased. This change in weight is due to the movement of water through the semipermeable membrane of the potato cells through osmosis.
In conclusion, the effect of salt concentration on osmosis in potato cells is significant, as it can cause the cells to either lose or gain water depending on the concentration of salt in the surrounding solution. Understanding this process is important for various applications, including the preservation of food, the understanding of biological systems, and the treatment of medical conditions.