Webspectra ucla. Introduction to IR Spectra 2022-12-19
Webspectra ucla
Rating:
5,2/10
421
reviews
Webspectra UCLA is a web-based platform developed by the UCLA Department of Chemistry and Biochemistry that allows users to access and analyze infrared (IR) and nuclear magnetic resonance (NMR) spectra. The platform is designed to be user-friendly and accessible to students, educators, and researchers, providing a valuable resource for understanding the structure and properties of chemical compounds.
IR spectra are used to identify functional groups in a molecule, and can be used to predict the physical and chemical properties of a compound. NMR spectra, on the other hand, provide information about the arrangement of atoms in a molecule and can be used to determine the structure of a compound. By analyzing both IR and NMR spectra, users can gain a comprehensive understanding of the structure and properties of a chemical compound.
Webspectra UCLA offers a wide range of features and tools to help users analyze and interpret spectra. Users can search for specific spectra or browse through a library of commonly-used compounds. The platform also includes a range of interactive tools, such as peak picking and integration, which allow users to extract key information from the spectra. In addition, Webspectra UCLA provides a range of educational resources, including tutorials and interactive exercises, to help users learn more about the principles and techniques of spectroscopy.
Overall, Webspectra UCLA is a valuable resource for anyone interested in learning more about the structure and properties of chemical compounds. By providing access to a wide range of spectra and interactive tools, the platform helps users gain a deeper understanding of the underlying principles of spectroscopy and apply this knowledge to a range of real-world applications.
IR Absorption Table
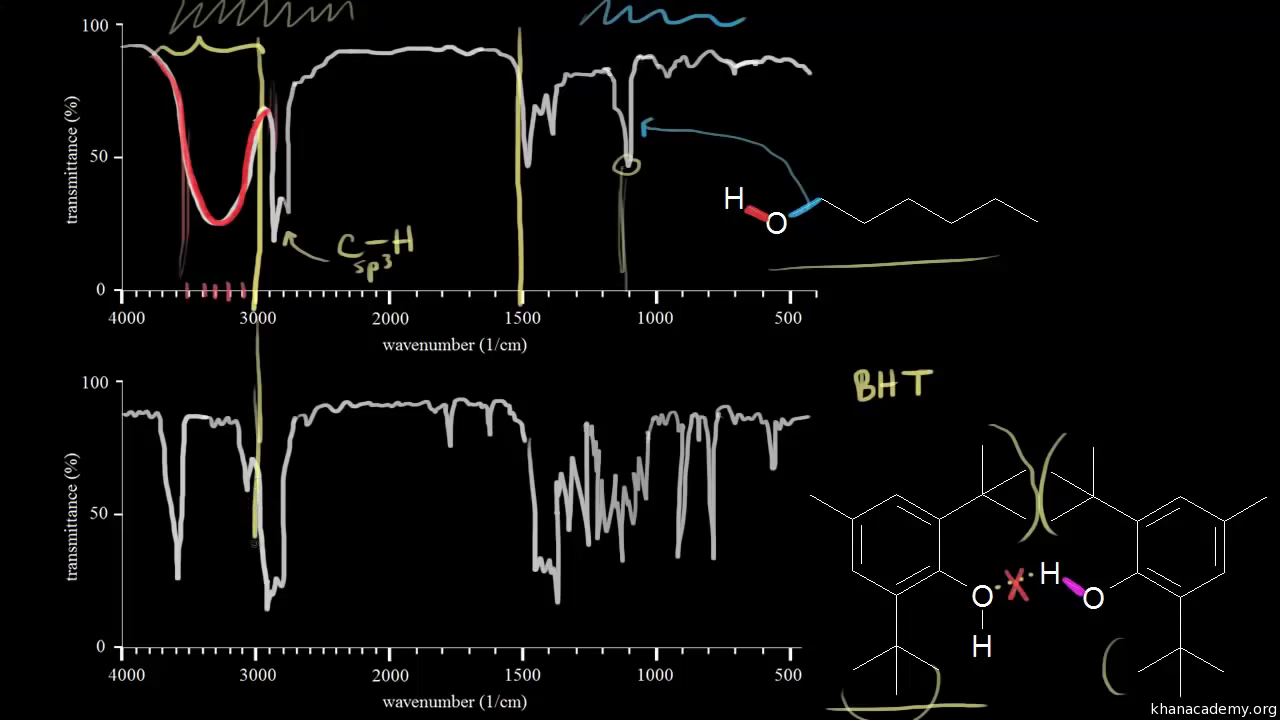
Other image formats are usually more suitable for smaller images such as pictures, and have problems dealing with the large spectrum images. Data was acquired on a Nicolet 510P FT-IR 7 and saved as raw XY plot data. UCLA Radiology is committed to providing outstanding patient care by combining excellence in clinical imaging, research, and educational programs with state-of-the-art technology. This final image format, in which both types of one-dimensional spectra are stored on the WWW server, is a non-standard, compressed image format designed specifically for WebSpectra. JavaScript code is embedded in the HTML shown to the user that dynamically replaces the region under the cursor with this second set of "highlighted" sections.
Next
Radiology & Imaging
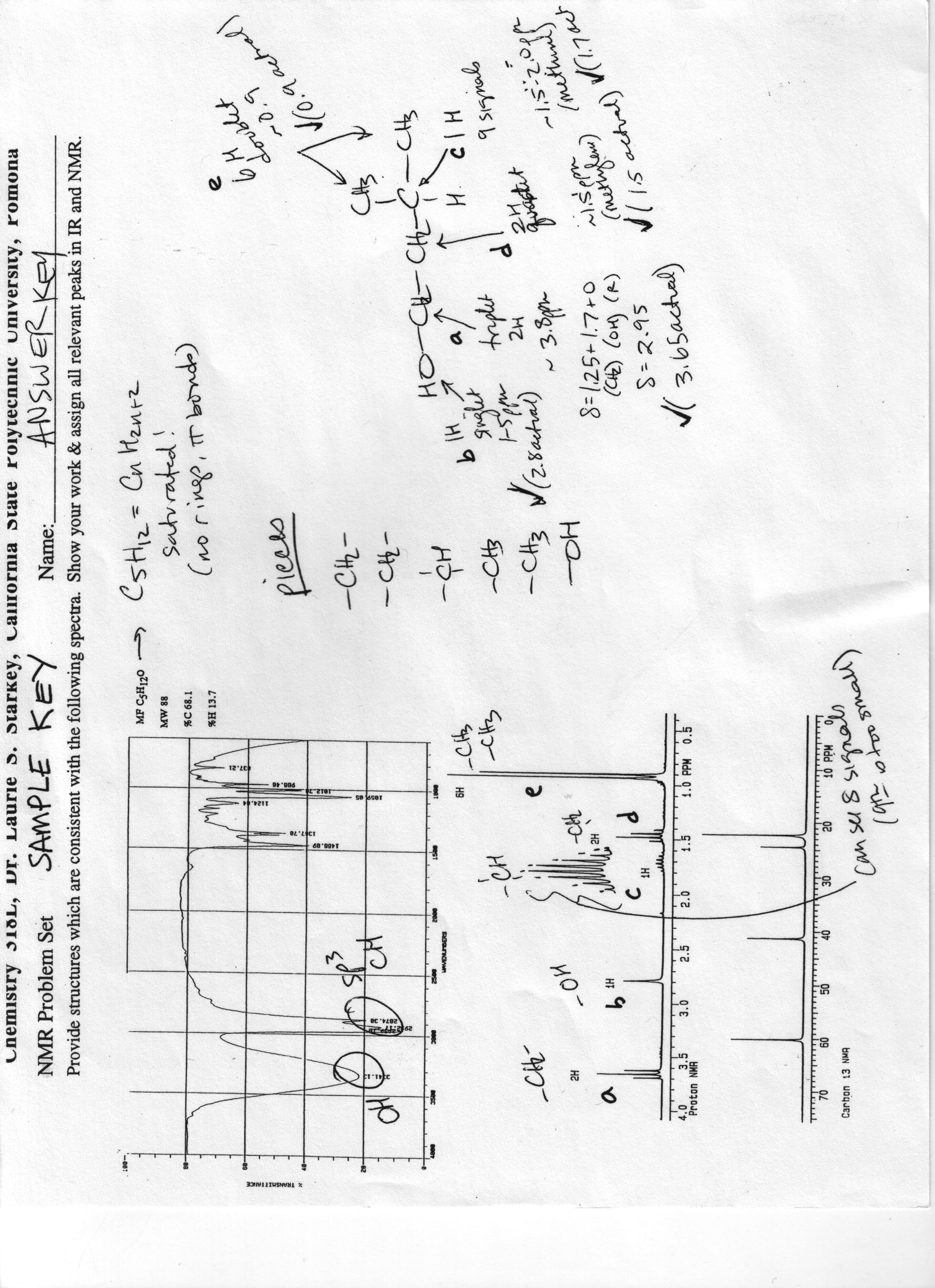
Limit to the methods you want to view. For DEPT spectra, the four individual spectra O o, 45 o, 90 o, 135 o were combined into one image at this point as well. A broad signal shape is sometimes indicated by br. The greater the masses of attached atoms, the lower the IR frequency at which the bond will absorb. We maintain strong interdisciplinary links with other departments within the Medical Center, and the UCLA campus. In these situations the broad O-H peak is replaced by a sharp signal around 3600 cm -1.
Next
Welcome to Virtual Office Hour
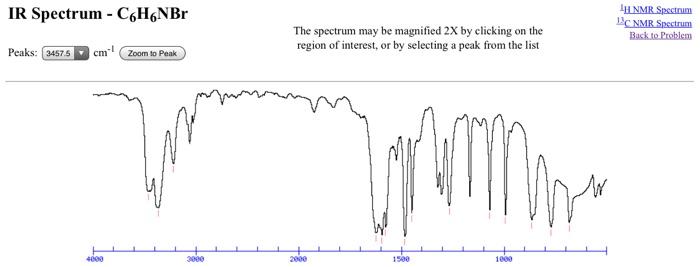
The manipulation of 2D NMR spectra will be discussed later. Dynamic presentation of spectra in the GIF format is made practical through the integration of a common gateway interface CGI program and JavaScript into the WWW site. Welcome to UCLA Radiology Scheduling and Access for your diagnostic and interventional studies. The UCLA radiology department faculty and research programs are internationally recognized. Radiation in this region can be utilized in organic structure determination by making use of the fact that it is absorbed by interatomic bonds in organic compounds. The Effects of Mass on Frequency As mentioned previously, one of the major factors influencing the IR absorption frequency of a bond are the identity of the two atoms involved.
Next
UCLA

Miller Department of Chemistry and Biochemistry University of California, Los Angeles, California 90095-1569 Display Issues Given the digital nature of modern spectroscopic data such as NMR and IR spectra, it would seem obvious to use the WWW to present this information. An Example IR Spectrum Because each interatomic bond may vibrate in several different motions stretching or bending , individual bonds may absorb at more than one IR frequency. Consequently tables of IR absorptions are arranged by functional group -- it some versions these may be further subdivided to give more precise information. If you would prefer, you may set your browser to refuse cookies or alert you when cookies are being sent; however, it is possible that some parts of our sites will not function properly if you do so. . The signal frequencies are generated from the Bruker xwinnmr software mentioned in the initial step in NMR processing.
Next
WebNMR Paper

By creating a very dilute solution of the sample, or acquiring the IR spectra in the gas phase, hydrogen bonding is prevented through lack of molecular contact. Amide N-H Stretch 3700 - 3500 m As with amines, an amide produces zero to two N-H absorptions depending on its type. Processing of IR spectral data that brings IR spectra to the final storage format is, in comparison to the NMR spectra, much simpler. Although the spectral images are the most important component of the WebSpectra database, there are several auxiliary files that were also generated during data processing. For example, neither of the carbon-carbon bonds in ethene or ethyne absorb IR radiation.
Next
Introduction to IR Spectra
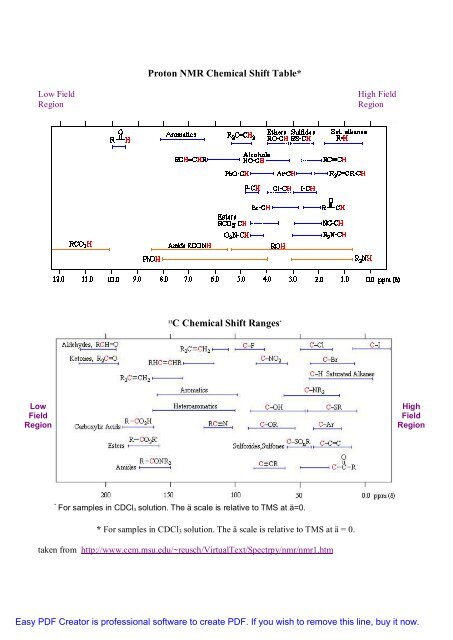
One of the important points in processing and storage of spectra is the preservation of the high-resolution data. By spanning a greater area, spectral accuracy is maintained even at the low resolution per unit area. Note: no spectra, but references and links to journal articles where you will find spectra. When the user selects one of these regions, the CGI handles finding and delivering the new and more magnified spectral section. IR Absorption Table Table of IR Absorptions Functional Group Characteristic Absorption s cm -1 Notes Alkyl C-H Stretch 2950 - 2850 m or s Alkane C-H bonds are fairly ubiquitous and therefore usually less useful in determining structure. Thus as an IR spectrum is acquired IR absorptions will occur at varying frequencies for each of these bonds. This results in unacceptable data loss, so the solution was to expand spectra across multiple "pages" within the Postscript file.
Next
WebSpectra

Chemical bonds in different environments will absorb varying intensities and at varying frequencies. The important issue on this latter point is that spectral magnification is principally a one-dimensional function, yet no WWW browser or plug-in supports this type of image manipulation. At this point, the only way to store the fully processed spectra is in Postscript format 4. As a general rule, the most important factors determining where a chemical bond will absorb are the bond order and the types of atoms joined by the bond. At this point, the vertical resolution is reduced to 36 dpi to reduce file size and increase computational speed, since extreme precision is not needed in that direction. First, alkane C-H stretching absorptions just below 3000 cm -1 demonstrate the presence of saturated carbons, and signals just above 3000 cm -1 demonstrate unsaturation.
Next
Spectra
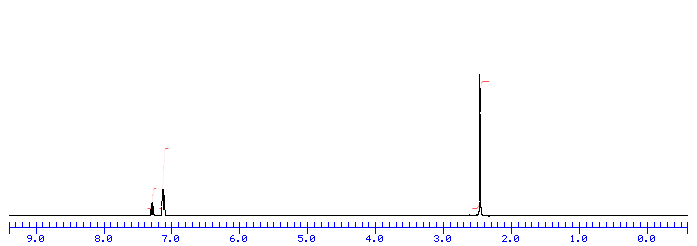
A very broad peak in the region between 3100 and 3600 cm -1 indicates the presence of exchangeable protons, typically from alcohol, amine, amide or carboxylic acid groups see further discussion of this below. The SPC format solves these barriers by storing data in sections, such that it is possible to jump immediately to any specific portion of the image, dramatically decreasing access time since only the necessary data is retrieved. Returning to 2D NMR spectra such as COSY, these are stored as multiple GIF images. At this point, the processing of 1D NMR and IR spectra converge Figure , and that of 2D NMR spectra diverges from those two. These are displayed beside the spectrum and allow calculation of coupling constants as well as location of signals and magnification. By fine-tuning the properties of the SPC image format, it has been possible to minimize access speed to the point where the WWW delivery is seamless. Introduction to IR Spectra Introduction to IR Spectra Theory An invaluable tool in organic structure determination and verification involves the class of electromagnetic EM radiation with frequencies between 4000 and 400 cm -1 wavenumbers.
Next
WebSpectra: Online NMR and IR Spectra for Students
This strong collaborative interaction between research and clinical practice allows us to rapidly implement new, and sometimes revolutionary, imaging and therapeutic innovations. This conversion is relatively straightforward, although one PCX file is produced for each of the virtual pages in the Postscript file. The WebSpectra CGI and JavaScript programs solve the problems of size and resolution by presenting "only what is needed" at any one time. In most cases, especially at higher magnifications, this is unnecessary, since only a small portion of that data is actually being displayed. In addition, each section is individually compressed, so the SPC format produces files that are approximately 40 times smaller than the raw data. All of the proton and carbon NMR spectra on the site were obtained using a Bruker ARX400 spectrometer 1.
Next
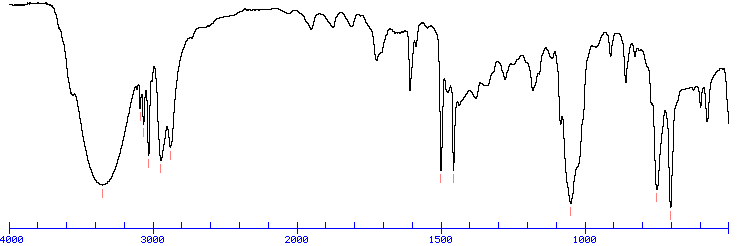
Through this intricate processing scheme, it is possible to present students high quality, but simple and manageable spectral images which allow them to focus on the most important issue - solving the problems at hand. Each spectra is divided into several regions and stored at multiple magnification levels. These data can be quickly referenced through tables of IR absorption ranges and compared to the spectrum under consideration. Thus, with the SPC format it is practical not only to deliver spectra at high resolution, but also to store a large number of such spectra in a relatively small amount of disk space. The second is illustrative of this property that heavier atoms deuterium vs. Therefore the same or similar functional groups in different molecules will typically absorb within the same, specific frequency ranges. Stretching absorptions usually produce stronger peaks than bending, however the weaker bending absorptions can be useful in differentiating similar types of bonds e.
Next









