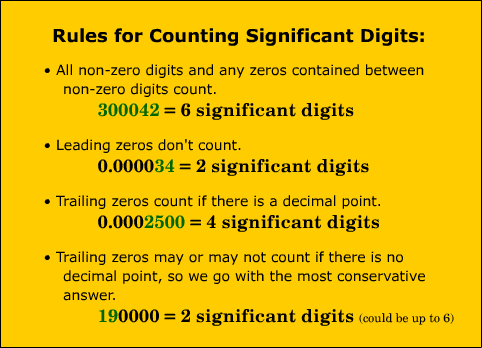
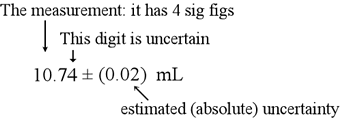Technology has become an integral part of our daily lives. From the smartphones in our pockets to the computers on our desks, technology has revolutionized the way we communicate, work, and access information.
One of the major benefits of technology is the way it has connected us globally. With the internet and social media, we can connect with people across the world and share ideas, opinions, and experiences. This has led to a more connected and informed global community.
Technology has also changed the way we work. With the advent of laptops and cloud computing, we can now work from anywhere and at any time. This has led to a rise in remote work and the gig economy, giving people more flexibility in their careers and allowing them to pursue their passions and interests.
In addition, technology has made it easier for people to access information and learn new things. With the internet and online educational resources, we can learn about any topic at any time and from any location. This has opened up new opportunities for learning and personal growth.
However, technology also has its drawbacks. One major concern is the issue of privacy. With the amount of personal information we share online, there is a risk of data breaches and identity theft. In addition, the increasing reliance on technology has led to a decrease in face-to-face communication and a rise in screen time, which can have negative impacts on mental health and social skills.
Overall, technology has brought about many positive changes in our lives, but it is important to use it responsibly and consider the potential negative impacts. It is up to us as individuals and as a society to find a balance and use technology in a way that benefits us and the world around us.