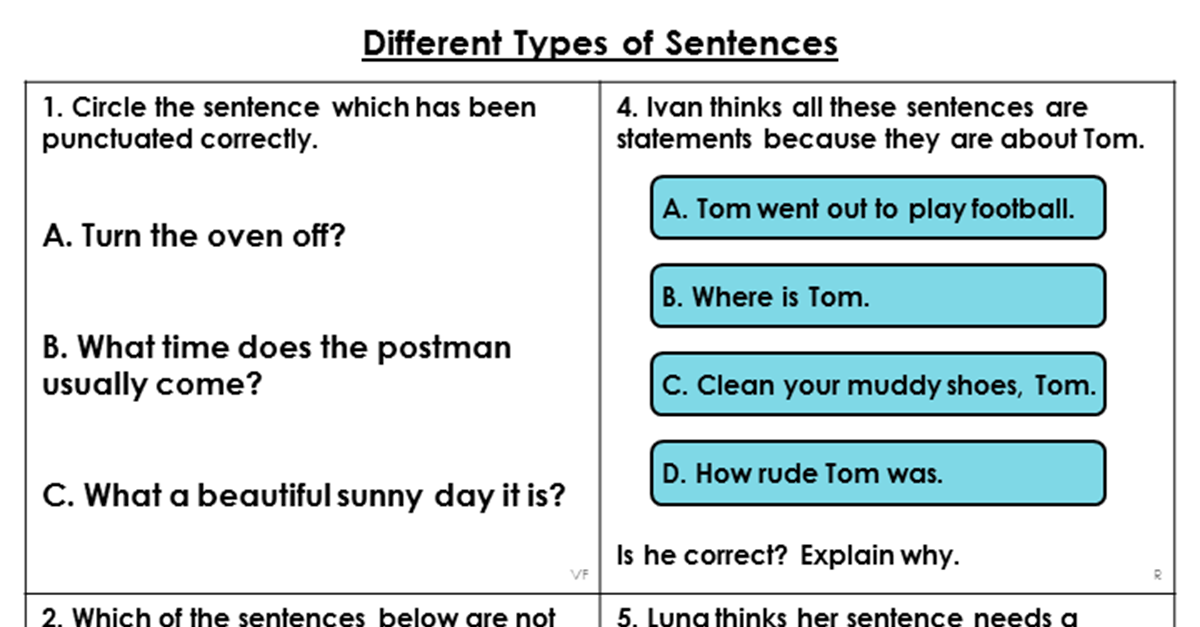
Gravimetric analysis is a method of quantitative chemical analysis in which the mass of a compound is used to determine its quantity. This technique is particularly useful for determining the concentration of a soluble chloride, such as sodium chloride (common table salt). In this essay, we will discuss the general principles of gravimetric analysis and the specific steps involved in performing a gravimetric analysis of a soluble chloride.
The basic principle behind gravimetric analysis is the measurement of mass. In order to determine the mass of a compound, it must first be isolated from the rest of the sample. This is typically done through a process called precipitation, in which the compound is transformed into a solid that can be easily separated and weighed.
The specific steps involved in a gravimetric analysis of a soluble chloride depend on the particular chloride being analyzed and the desired end result. However, there are some general steps that are followed in most gravimetric analyses.
First, the sample is prepared by dissolving it in a suitable solvent. The solvent should be chosen based on the solubility of the compound being analyzed and the desired end result. For example, water may be used as a solvent for a soluble chloride if the goal is to determine the mass of the chloride.
Next, the precipitating reagent is added to the sample. This reagent is chosen based on the solubility of the compound being analyzed and the desired end result. For example, a soluble chloride may be precipitated as a silver chloride by adding a silver nitrate solution to the sample.
Once the precipitate has formed, it is allowed to settle to the bottom of the container. The supernatant liquid is then carefully decanted, leaving the precipitate behind. The precipitate is then washed with a solvent to remove any impurities that may have been present in the sample.
Finally, the precipitate is dried and weighed to determine its mass. This mass can then be used to calculate the concentration of the soluble chloride in the original sample.
In summary, gravimetric analysis is a powerful tool for determining the concentration of a soluble chloride. By following the steps outlined above, it is possible to accurately and precisely determine the mass of a compound, which can be used to calculate its concentration.