Informational writing is a type of writing that is designed to inform, educate, or explain a particular topic to the reader. It is often used in non-fiction writing, such as textbooks, encyclopedias, and academic papers. It can also be found in magazines, newspapers, and online articles.
There are many ideas for informational writing, and the topic you choose will depend on your audience and your purpose for writing. Here are a few ideas to get you started:
Write about a historical event or person: This could be a famous battle, a significant figure in history, or a significant moment in time.
Write about a scientific concept or discovery: This could be something related to physics, biology, or any other scientific field.
Write about a current event or issue: This could be something that is happening in the world today, such as a natural disaster, a political crisis, or a social issue.
Write about a cultural tradition or practice: This could be something related to a particular country, region, or group of people, such as a holiday, a religious ceremony, or a cultural tradition.
Write about a hobby or interest: This could be something that you are passionate about, such as cooking, gardening, or playing a sport.
No matter what topic you choose for your informational writing, it is important to do thorough research and present the information in a clear and concise manner. Make sure to include relevant facts and details, and use sources to support your arguments. Additionally, it is important to consider your audience and tailor your writing to their needs and interests. By following these tips, you can effectively inform, educate, and explain to your readers through your writing.
What is a period on the periodic table

How do you find the group number of F block elements? What are the 4 most common atoms? Solution : a The elements belonging to the same period have same number of shells. The first two groups are 1A and 2A, while the last six groups are 3A through 8A. Which of these elements is a member of the same group? Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type. For example, in group 1, lithium Li is the smallest element, whereas francium Fr is the largest element. What are the 7 groups of the periodic table? What charge do group 4 elements have? The number of electrons in the outermost shell of an atom grows from 1 to 8 as the electronic configurations of elements vary over time. There are seven periods in the periodic table, with each one beginning at the far left.
6.4: Modern Periodic Table
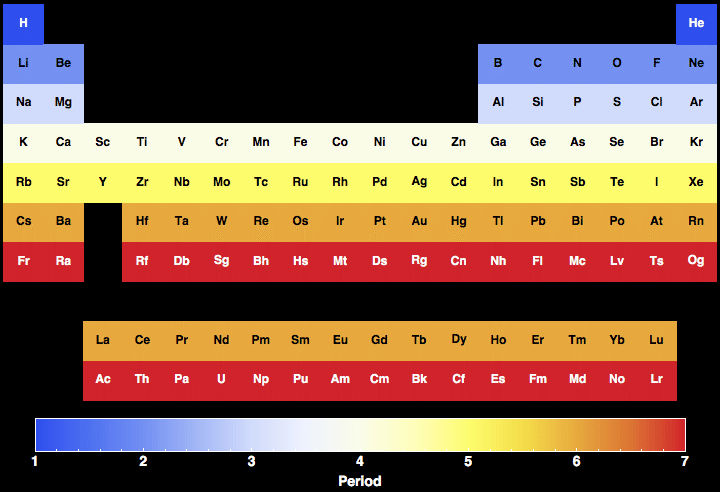
Elements in the same group share common chemical and physical properties. Group 4A or IVA of the periodic table includes the nonmetal carbon C , the metalloids silicon Si and germanium Ge , the metals tin Sn and lead Pb , and the yet-unnamed artificially-produced element ununquadium Uuq. As a result, as a period progresses from left to right, the tendency of atoms to lose electrons decreases. For example, it is found that Al and Beare amphoteric elements with almost the same properties by the diagonal rule. There are total 18 vertical columns on periodic table. Answer: The number of valence electrons of elements in the group are the same.
Period (Periodic Table)

Sample Questions Question 1: Which element has the largest size in the third period? Read more about starting periods. What is period 4 on the periodic table? Electronic configurations are similar among elements with similar chemical properties. As a result, sodium is the most electropositive element in the third period, whereas chlorine is the most electronegative. The electronic configurations of elements show regular periodicity when atoms are organised by increasing atomic numbers. The 4d and 5d elements exists higher oxidation state than 3d elements.
What is groups and periods in periodic table?

The common feature is that the atoms of all elements consist of electrons, protons, and neutrons. Metals in the third period include sodium, magnesium, and aluminium. Periods in periodic table are the horizontal rows on the periodic table. What is the energy level of the period 4 transition elements? These publications may be in print, as is thelawdictionary below, or they may be electronic. What are 4d elements? These properties of periodic table elements are discussed in further detailbelow.
The Periodic Table of Elements Explained
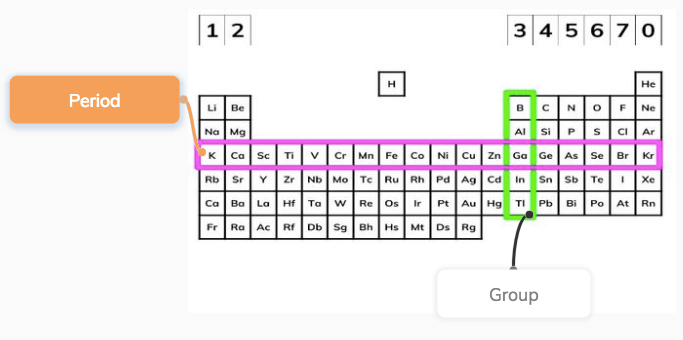
Most recent depictions of the periodic table show an incomplete seventh period. There are two different numbering systems that are commonly used to designate groups, and you should be familiar with both. Hence there are 18 groups. Since the elements of group 2 are on the left side of the table, so the given element is a metal. Many new elements have been discovered, while others have been artificially synthesized. The period number is related to the number of electron occupied shells in the element and the period number is linked to its valence electrons.
Periods in Periodic Table (Explained with Images)

The scientists place the members with the same electron shell number in the same horizontal rowas a period See Figure 1. The elements in this group are also known as the chalcogens or the ore-forming elements because many elements can be extracted from the sulphide or oxide ores. However, there are exceptions, such as chromium. Periods on the Periodic Table. Uranium has been used in this manner to produce elements 93-100. Elements of period 2 and period 3 are given in the tables below. The atomic number of each element is written above the symbol.
Periodic Table Groups and Periods

Phosphorus, sulphur, and chlorine are non-metallic elements. The number of elements in each time varies. The present periodic table is said to have been invented by Bohr. The middle groups use B in their titles. How many groups are present in D-Block? What are the 7 periods of the periodic table? As a result, losing valence electrons is difficult. Atoms in a group share the same number of valence electrons. Answer: The 4d elements are larger in size as compared to 3d elements.
What is period 4 on the periodic table?
Each square shows the chemical symbol of the element along with its name. Many of these binary compounds are nonstoichiometric and exhibit metallic conductivity. The element on the left in the third period is sodium, hence sodium Na has the largest size in the third period. Do group 4 elements form ions? Question 2: What is the tendency to lose electrons over aperiod? For instance, the metallic nature of group 1 increases from lithium to francium. What is common in all elements? A group is a vertical column of the periodic table, based on the organization of the outer shell electrons. Size of atoms The size of atoms or atomic size grows as one moves down a group of the periodic table. Period that Uranium is found.



 Periodic groups and periods.jpg)



