What is the common name for calcium hydroxide solution. Calcium Hydroxide in Food: Pickling and Other Uses, Plus Safety Tips 2023-01-01
What is the common name for calcium hydroxide solution
Rating:
7,9/10
1328
reviews
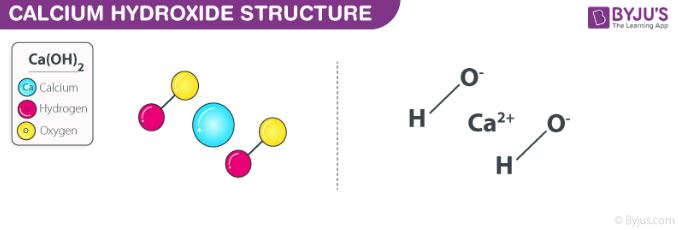
Calcium hydroxide, also known as slaked lime, is a chemical compound with the formula Ca(OH)2. It is a white, alkaline, crystalline solid that is soluble in water. When dissolved in water, it forms a solution known as lime water or calcium hydroxide solution.
Lime water is commonly used in a variety of industrial and household applications. In industry, it is used as a pH adjuster, a water treatment agent, and a chemical intermediate. It is also used in the production of cement, glass, and paper. In households, lime water is often used as a cleaning agent, particularly for removing stains and discoloration from surfaces.
One of the most well-known uses of lime water is in the treatment of drinking water. When added to water, it reacts with dissolved carbon dioxide to form calcium carbonate, which helps to remove impurities and improve the taste of the water. Lime water is also used in the treatment of wastewater, as it helps to neutralize acidic substances and remove impurities.
In addition to its practical uses, lime water has a long history of medicinal use. It was once believed to have numerous health benefits, including the ability to treat a variety of ailments such as sore throat, indigestion, and skin infections. While some of these traditional medicinal uses have been largely abandoned, lime water is still used today as an antacid and a mild laxative.
In conclusion, the common name for calcium hydroxide solution is lime water. It is a versatile chemical compound that is used in a variety of industrial and household applications, as well as in the treatment of drinking water and wastewater. It has a long history of medicinal use and is still used today as an antacid and laxative.
Calcium Hydroxide

All Answers 5 Calcium Hydroxide is used a cavity liner, cement base, root canal filling material, direct and indirect pulp capping material in restorative dentistry. More safety Information: Concentrated Sodium Hydroxide, Calcium Hydroxide, Magnesium Hydroxide and Potassium Hydroxide are strong irritants and corrosive to the skin, eyes, respiratory tract and gastrointestinal system if ingested. The pozzolanic reaction is the chemical reaction that occurs in portland cement upon the addition of pozzolans. How much calcium to take pregnant? Archived from PDF on 25 March 2012. Sodium hydroxide is found in drain cleaner.
Next
What is calcium hydroxide base?
Dycal® Calcium Hydroxide Liner is a two-component, rigid-setting, self-curing material designed for use in direct and indirect pulp capping and as a protective liner under dental adhesives, varnishes, filling materials, cements, and other base materials. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E526. Calcium causes the heart and arteries to squeeze contract more strongly. Used in alkaline batteries. So it's no surprise that I eventually became a teacher. Calcium hydroxide, also known as slaked lime with the chemical formula Ca OH 2 is a source of hydroxide ions when dissolved in aqueous solutions. Retrieved 18 June 2021.
Next
14.3: Bases
The pH of Dycal ® and PCFA ranged between 9. Inhaling lime dust may lead to irritation of breathing passages, coughing and sneezing. Why fly ash is used? See also How much honey from warre hive? These include soaking vegetables in ice water for four to five hours before pickling or using pickling salt. Does calcium hydroxide react with plastic? What is calcium hydroxide used for? An increase in the hydroxyl ion concentration causes further silica dissolution from the reactive aggregate, which leads to the formation of calcium alkali silicate hydrates CASH. Therefore, this compound is a base.
Next
What is the role of calcium hydroxide in cement?

What is the pozzolanic reaction link up with the chemical reaction? What is the correct formula for calcium hydroxide? This can lead to severe injury or death. Fly ash use in concrete improves the workability of plastic concrete, and the strength and durability of hardened concrete. This is because pickling lime has been linked to A number of recipes offer alternatives to keep your pickles crunchy. It increases calcium levels by targeting the skeleton, the kidneys, and the intestine. This material also helps in the formation of reparative dentine.
Next
Calcium hydroxide

What is calcium hydroxide dilute slurry? How are calcium levels regulated by hormones? What is lime water formula? Is calcium hydroxide acid or base? Fly ash is a fine powder that is a byproduct of burning pulverized coal in electric generation power plants. Feel free to ask your doubts through the comment box below if any. But in general, they are known by their common name and many of them are a part of our day to day life. Is calcium carbonate the same as calcium hydroxide? What are the uses of calcium hydroxide? Calcium hydroxide is an odorless white powder. Thus, according to 2, and so increases its solubility at low temperature. Important Chemical Compounds List and their Formulas Sl.
Next
What is the common name for calcium hydroxide solution?

Your heart, muscles and nerves also need calcium to function properly. Adding lime to the soil in autumn is the easiest answer to how to raise calcium in the soil. PDF on 31 October 2007. However, if you work with industrial-grade calcium hydroxide, ingesting it can result in calcium hydroxide poisoning. Food-grade calcium hydroxide is generally safe. Chemical Principles 6th Ed. Most patients with hyperparathyroidism have calcium levels that fluctuate from high to slightly high, to high-normal.
Next
What is the Common Name of Calcium hydroxide solution ?
2Xray.jpg/220px-Mg(OH)2Xray.jpg)
How to improve the calcium content of garden soil? Now let us have a look at the common names and formulas of some important chemical compounds. See also Why child class reference cannot hold parent object? Is lime poisonous to humans? Low amounts of this vitamin can not only make your bones weaker but can also cause muscle contraction and eye twitches. What does calcium hydroxide do to teeth? How does Dycal work? The placement of calcium hydroxide should be followed by a layer of RMGI to protect it from its drawbacks. What is pozzolanic property? Can calcium levels go up and down? Does calcium hydroxide cure light? Retrieved 12 May 2022. Does calcium hydroxide react with stainless steel? Then insulin and glucose are given, which move potassium from blood into cells, thus lowering the potassium level in blood. Calcium hydroxide, Ca OH 2, is sparsely soluble at room temperature in water 1.
Next
Calcium Hydroxide in Food: Pickling and Other Uses, Plus Safety Tips
Plaque in the arteries can cause heart attacks. What is calcium hydroxide in concrete? Many bases, like soaps, are slippery to the touch. What is a ct calcium score test? Calcium hydroxide, also called slaked lime, Ca OH 2, is obtained by the action of water on calcium oxide. As a young girl, I was always fascinated by the world around me. What is leaching of calcium? PTH is released in response to low blood calcium levels.
Next
Chemical Compounds List, Common Names, Formulas

The subscript that lies to the right of the symbol for each element indicates the number of atoms of that element that make up that chemical compound. But because of tobacco consumption, the practice has been discouraged. Stainless Steel 304 Stainless Steel 316 Calcium Hydroxide B-Good B-Good Calcium Hypochlorite C-Fair B-Good Calcium Nitrate C-Fair B-Good Why are root canals done in 2 visits? Leaching of calcium ions increases the porosity of cement-based material, thus resulting in degradation including damage to the pore structure. The goal of endodontic treatment is the prevention and control of pulpal and periradicular infections. What is the common name for calcium hydroxide solution? Dissolution des acides et des alcalis. If ingested, lime can cause pain, vomiting, bleeding, diarrhea, a drop in blood pressure, collapse, and in prolonged cases, it can cause a perforation of the esophagus or stomach lining. When a compound is made up of a metal cation and a polyatomic anion, the compound is considered to be ionic.
Next
is the common name of calcium hydroxide.

Is calcium hydroxide corrosive to metal? Limewater is the common name for a dilute aqueous solution of calcium hydroxide. In the laboratory calcium hydroxide can be prepared by mixing aqueous solutions of calcium chloride and sodium hydroxide: CaCl 2 + 2 NaOH Ca OH 2 + 2 NaCl When heating calcium hydroxide to 512 °C the calcium hydroxide decomposes into calcium oxide and water: Ca OH 2 CaO + H 2O Ca OH 2 is only slightly soluble in water 0. Fortified fruit juice Fruit juices are sometimes If you want to use pickling lime for home canning, make sure you thoroughly rinse it off vegetables before canning them to avoid any botulism risks. It also has medical and dental uses. When mixed with water, a small proportion of it dissolves, forming a solution known as limewater, the rest remaining as a suspension called milk of lime. Dycal contributes to the partial closure of the pulp exposure, producing a defective reparative dentinal bridge. Is calcium hydroxide safe for skin? Therefore, this compound is a base.
Next






2Xray.jpg/220px-Mg(OH)2Xray.jpg)


