Zinc iodide is an inorganic compound with the chemical formula ZnI2. It is a white, crystalline solid that is highly soluble in water and forms a strong, acidic solution when dissolved.
The formula for zinc iodide can be written in a few different ways. One way to write the formula is simply as ZnI2, which represents the elemental composition of the compound. This indicates that the compound is composed of one atom of zinc (Zn) and two atoms of iodine (I).
Another way to write the formula for zinc iodide is to include the charge of the ions present in the compound. Zinc iodide is an ionic compound, which means that it is composed of charged ions rather than neutral atoms. In this case, the zinc ion is a cation with a charge of +2, while the iodine ion is an anion with a charge of -1. The formula for zinc iodide can therefore also be written as Zn^2+I^-2, which represents the ionic charges of the atoms in the compound.
In addition to these two ways of writing the formula for zinc iodide, it can also be written in a chemical equation, which represents a chemical reaction. For example, the reaction between zinc and iodine can be written as:
Zn + I2 → ZnI2
This equation shows that when zinc and iodine are combined, they react to form zinc iodide. The reactants, or starting materials, are shown on the left side of the arrow, while the product, or end result, is shown on the right side.
In summary, the formula for zinc iodide can be written as ZnI2, Zn^2+I^-2, or in a chemical equation. It is an inorganic compound composed of one atom of zinc and two atoms of iodine, and is highly soluble in water.
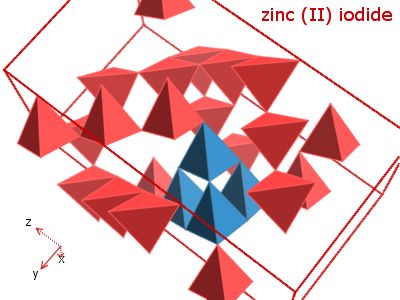



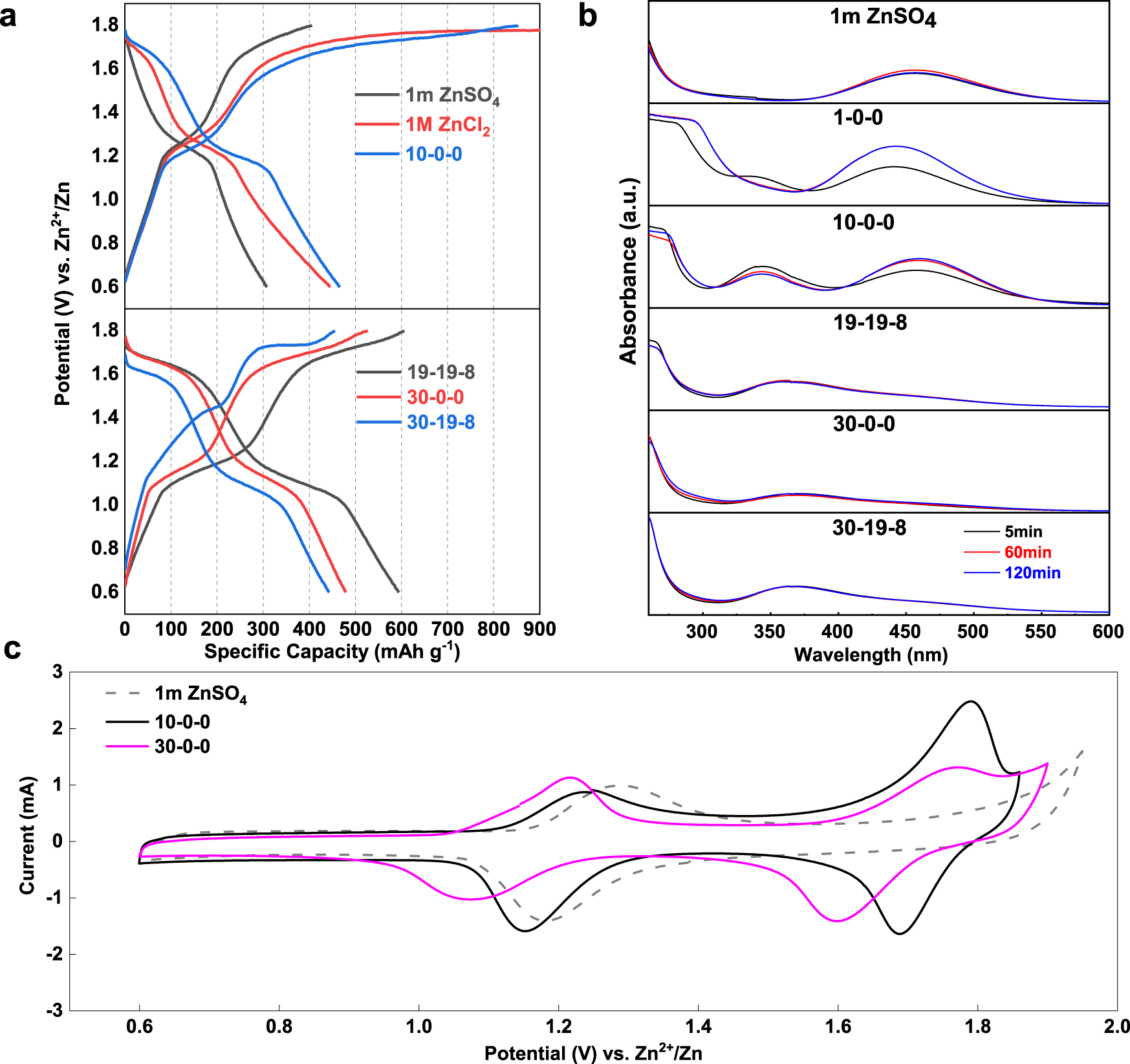
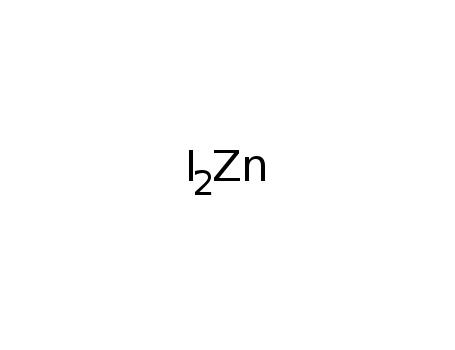


.png/220px-Portion_of_ZnI2_lattice_(ICD_Code2404).png)