Catalase is an enzyme found in the cells of many living organisms, including plants, animals, and microorganisms. It plays a vital role in the breakdown of hydrogen peroxide, a toxic byproduct of metabolic reactions that can damage cells if left unchecked.
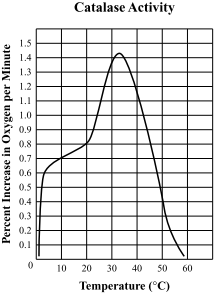
The activity of enzymes, including catalase, is strongly influenced by temperature. In general, as the temperature increases, the rate of enzyme-catalyzed reactions increases as well. However, there is a point at which the enzyme becomes denatured, or loses its ability to function properly, due to the effects of extreme heat.
The optimum temperature for catalase activity is around 37°C, which is close to the normal body temperature of mammals. At this temperature, catalase is able to function at its maximum rate, breaking down hydrogen peroxide quickly and efficiently.
At temperatures below the optimum, the rate of catalase-catalyzed reactions decreases as the enzyme becomes less active. This is due to the fact that the lower temperature slows down the movement of the enzyme's catalytic site, which reduces the frequency of successful collisions between the enzyme and substrate (in this case, hydrogen peroxide).
At temperatures above the optimum, the rate of catalase-catalyzed reactions also decreases due to denaturation of the enzyme. Extreme heat can cause the enzyme's protein structure to change, disrupting its active site and rendering it unable to function properly.
In summary, the optimum temperature for catalase is around 37°C, as this allows the enzyme to function at its maximum rate without becoming denatured. It is important to note that the optimum temperature can vary slightly depending on the specific organism and conditions in which the enzyme is functioning.