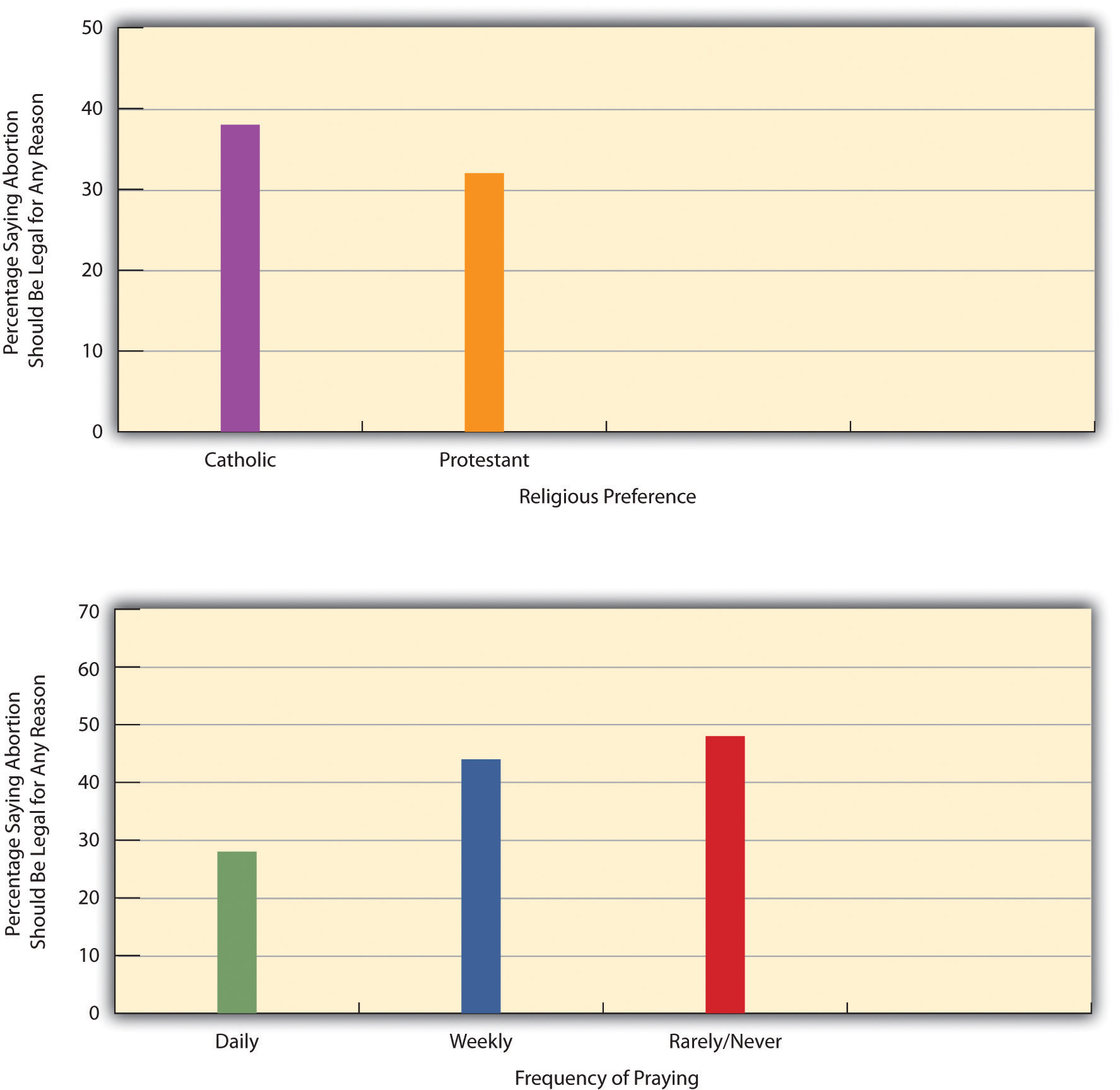
The pharmaceutical product life cycle refers to the stages that a pharmaceutical product goes through from its development to its withdrawal from the market. This process is crucial for the pharmaceutical industry as it helps companies to plan for the development, production, and marketing of their products.
The first stage of the pharmaceutical product life cycle is the research and development (R&D) phase. This stage involves the identification of a potential drug target, the design and synthesis of a compound that can bind to the target, and the testing of the compound in the laboratory to determine its effectiveness and safety. This phase can take several years and is typically the most expensive and time-consuming part of the product life cycle.
The next stage is the clinical development phase, which involves conducting clinical trials to determine the safety and efficacy of the drug in humans. Clinical trials are conducted in three phases: Phase 1 trials involve a small number of healthy volunteers and are designed to determine the drug's safety profile and dosage range. Phase 2 trials involve a larger group of patients and are designed to evaluate the drug's effectiveness and determine optimal dosage. Phase 3 trials involve an even larger group of patients and are designed to confirm the drug's effectiveness, monitor side effects, and compare the drug to existing treatments.
If the clinical trials are successful, the drug can then be submitted for regulatory approval to the relevant authorities, such as the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). This process can take several years and requires the submission of extensive data on the drug's safety, efficacy, and manufacturing process.
If the drug is approved, it moves into the commercialization phase, where it is manufactured and marketed to healthcare providers and consumers. This phase can last for several years, depending on the drug's patent protection and market demand.
Eventually, the drug will reach the end of its patent protection and face competition from generic versions. This can lead to a decline in sales and a decrease in the drug's profitability. In some cases, the drug may be withdrawn from the market due to safety concerns or a lack of demand.
In summary, the pharmaceutical product life cycle is a complex and multi-faceted process that involves several stages, from research and development to clinical trials and regulatory approval, before a drug can be commercialized and made available to patients. Understanding the product life cycle is essential for pharmaceutical companies as they plan for the development and marketing of their products.
A conflict of interest occurs when an individual or organization is faced with a choice between two competing interests, and it is not clear which one should be prioritized. This can happen in a variety of settings, including professional, financial, and personal relationships. In the professional context, a conflict of interest can arise when an individual has a financial or personal interest in a company or organization that may influence their decisions or actions in their professional capacity. For example, a doctor may have a financial stake in a pharmaceutical company and may be tempted to prescribe its medications over those of a competitor, even if the competitor's product is more effective or less expensive.
Conflicts of interest can also occur in the political realm. For example, a politician may have a financial interest in a company that stands to benefit from a policy decision they are advocating for. In this case, the politician's personal financial interests may influence their decisions and actions in their official capacity, rather than acting in the best interests of their constituents.
In the financial industry, conflicts of interest can arise when financial advisors or brokers have a financial stake in the products they recommend to their clients. For example, a financial advisor may receive a higher commission for selling one investment product over another, even if the second product is more suitable for the client's needs.
Conflicts of interest can also occur in personal relationships. For example, a parent may have a financial interest in their child's success, which could influence their decision-making when it comes to their child's education or career path.
It is important to recognize and address conflicts of interest, as they can undermine trust and integrity, and can lead to unethical or biased decision-making. In order to mitigate the potential for conflicts of interest, individuals and organizations can adopt codes of conduct or policies that outline acceptable and unacceptable behaviors, and establish procedures for disclosing and managing conflicts of interest.
For example, a company may require employees to disclose any financial interests they have in other organizations that may conflict with their work responsibilities. This allows the company to identify and address any potential conflicts of interest before they become a problem. In the professional context, individuals may be required to disclose any conflicts of interest before participating in certain activities, such as serving on a panel or reviewing a grant application.
In summary, conflicts of interest can arise in a variety of settings and can have serious consequences if left unaddressed. It is important for individuals and organizations to be aware of and address conflicts of interest in order to maintain trust and integrity, and to ensure that decisions are made in an unbiased and ethical manner.