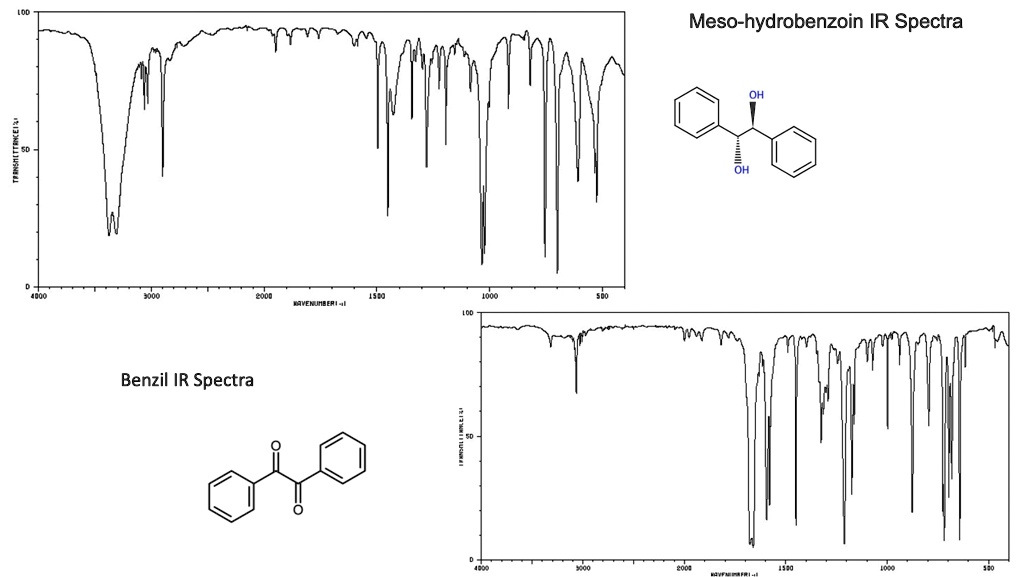
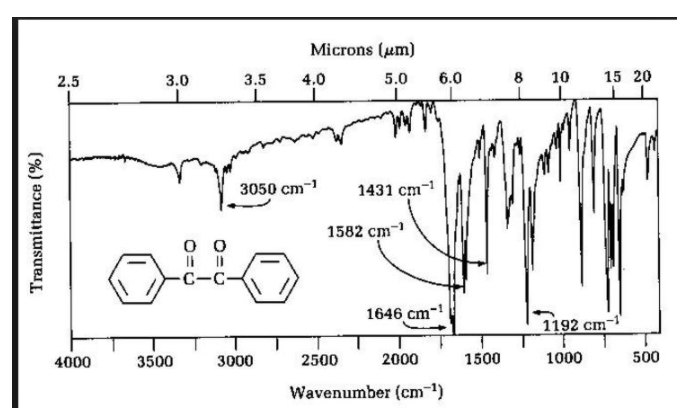
Infrared (IR) spectroscopy is a powerful analytical technique used to identify and characterize compounds based on their molecular structure. By measuring the absorption of infrared radiation by a sample, scientists can determine the functional groups present in the compound and identify the compound itself. In this essay, we will discuss the IR spectra of benzil, a diketone with the chemical formula C14H10O2.
The IR spectrum of benzil exhibits several key features that can be used to identify it. One of the most prominent features is the presence of absorption bands in the carbonyl region, between 1700-1750 cm-1. This is due to the presence of two carbonyl groups (C=O) in the molecule, which absorb infrared radiation at these wavelengths. The presence of these bands is a strong indication of the presence of a ketone or aldehyde functional group in the compound.
Another important feature of the IR spectrum of benzil is the presence of a broad absorption band in the fingerprint region, between 1000-1200 cm-1. This region is called the fingerprint region because it is highly sensitive to the specific molecular structure of the compound and can be used to differentiate between different compounds. In the case of benzil, the broad band in this region is due to the presence of aromatic C-H stretching vibrations.
In addition to these main features, the IR spectrum of benzil also exhibits absorption bands in other regions that can be used to confirm its identity. For example, there is an absorption band at 1620 cm-1 due to the presence of C=C double bonds, and an absorption band at 1450 cm-1 due to C-H in-plane bending vibrations. These bands are consistent with the known structure of benzil and can be used to confirm its identity.
In conclusion, the IR spectrum of benzil exhibits several key features that can be used to identify and characterize the compound. By measuring the absorption of infrared radiation by the sample, scientists can determine the functional groups present in the compound and confirm its identity. This makes IR spectroscopy a powerful tool for identifying and characterizing compounds in a wide range of applications.
benzil Table 13 Benzil Infrared Spectrum Analysis Peak Wave Number cm 1

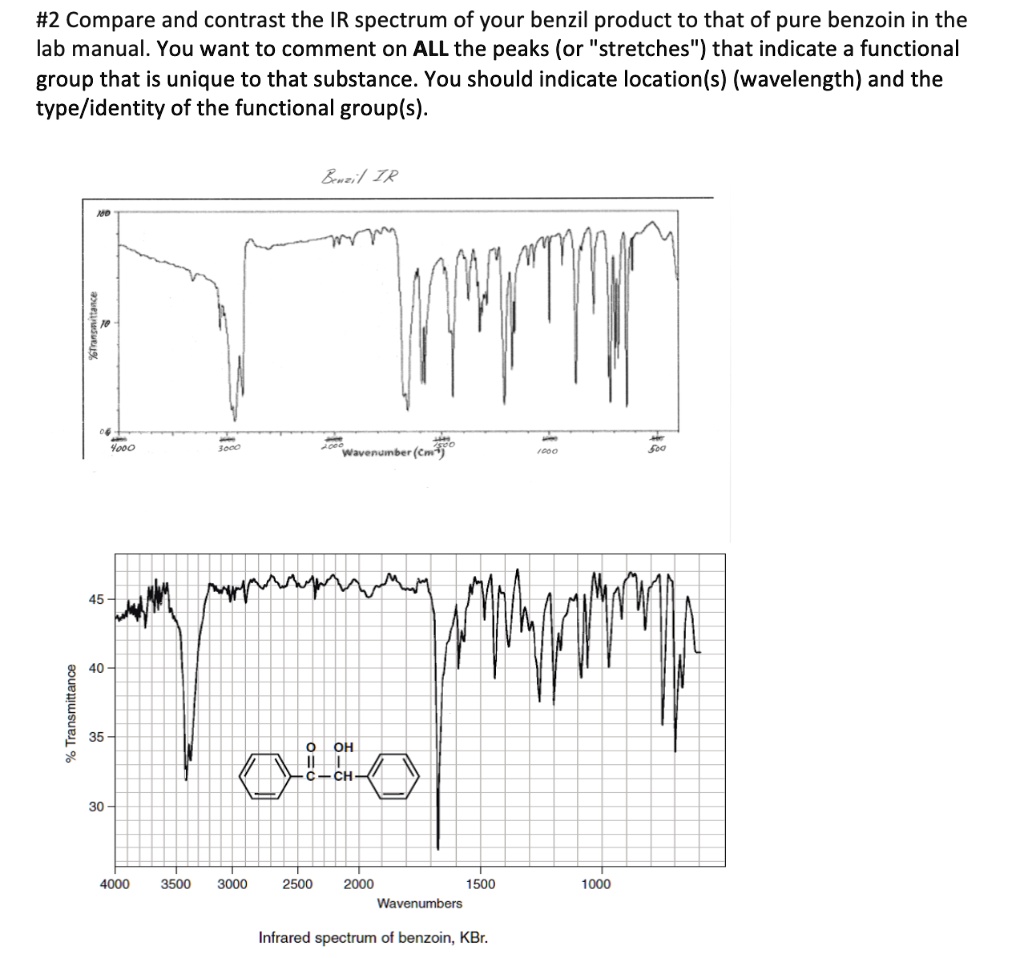
Peak assignments can be simplified by noting that 13C peaks tend to be larger if two carbons contribute to the absorption. The peak wavenumber listed above may have more than one functional group that is not listed because there are multiple functional groups per peak. This range is typically called the aromatic region of an 1H NMR spectrum. The red color proved the presence of nitric acid. The IR data may be saved as a JDX JCAMP Chemical Spectroscopic Data Exchange Format file. For OP, do you have any other data? Protons on carbons directly bonded to an aromatic ring, called benzylic protons, show up about 2. The peak at a little over 3000 cm -1 was not labeled on the IR spectrum,bu it corresponds with aliphatic hydrogen present one of the two aromatic rings.
IR Spectrum of Benzyl Alcohol(?) : chemhelp

How to Purchase First, make sure that the target chemical substance given below is the substance you need by checking the structure and other identifiers. Please flair yourself and read over the rules below before posting. It is OK if you are a little or a lot! The meta configuration's plane of symmetry mirrors two carbons of the benzene ring allowing for four aryl absorptions to occur. Please complete any questions as much as you can before posting. We will not do your homework for you, so don't ask. Answer The molecule contains two groups of equivalent protons: the twelve pointing to the outside of the ring, and the six pointing into the center of the ring. Substituted benzene rings have peaks that correspond to the substitution pattern mono, para, meta, etc.
IR Spectra for BENZIL

Functional groups will behave vibrate, stretch, flex, wiggle, basically move around at different ranges on the spectrum based on the type of functional group. It's difficult to determine a structure based on IR alone, unless you were given a list of organic compounds to choose from. I'd be deeply grateful if you can lend an hand. Only alkenes and aromatics show a C—H stretch slightly higher than 3000 cm -1. The important points to note about the proton NMR of aromatic compounds are the approximate chemical shifts of such protons and the complex splitting pattern that is sometimes observed. So, I did some of my dirty job and I think that I've received a Benzyl Alcohol spectrum. This is my first post on Reddit! Explain the unusual chemical shift of the latter peak.
15.4: Spectral Characteristics of the Benzene Ring
The ortho configuration has a plane of symmetry which mirrors each carbon in the benzene ring causing only three 13C aryl absorptions to occur. I don't have other data, but from what you all are saying now I'm very confident that I've in my hands the spectrum of a Benzyl Alcohol : Thanks again! It's difficult to pick out any obvious peaks here. MP, BP, NMR, etc? Agreed, I wouldn't worry about the peak at 700 cm-1. This is virtually the same range as nonaromatic alkenes 110-150 ppm so peaks in this region are not definitive proof of a molecule's aromaticity. Consequently, their 13C NMR spectra show six arene absorptions. .
Characterize the IR spectrum of methyl benzoate?

Due to the molecule's symmetry, the aryl protons appear as two doublets at 6. When the molecule is exposed to B 0, these p electrons begin to circulate in a ring current, generating their own induced magnetic field that opposes B 0. Infrared spectroscopy was used in order to determine the purity and overall identity of the synthesized product. The experiment yielded 1. Due to the decoupling in 13C NMR, the number of absorptions due to aromatic carbons can easily be observed.
In the induced field generated by the aromatic ring current, the benzylic protons are outside the ring — this means that the induced current in this region of space is oriented in the same direction as B 0. IR is mainly used to determine what functional groups are present, not necessarily the structure. This can be used to determine the relative positions ortho, meta, or para for di-substituted benzenes. It is also important that you describe the specific part of the problem you are struggling with. The end result is that benzylic protons, due to the anisotropy of the induced field generated by the ring current, appear to be highly de-shielded.