Benzoids, also known as benzoins, are a class of organic compounds that contain a benzene ring fused with an oxo group (-O-). Benzoin, specifically, is a white crystalline solid with a sweet, vanilla-like aroma. It is used in the production of fragrances, as a flavoring agent in food and beverages, and as a starting material in the synthesis of other chemicals.
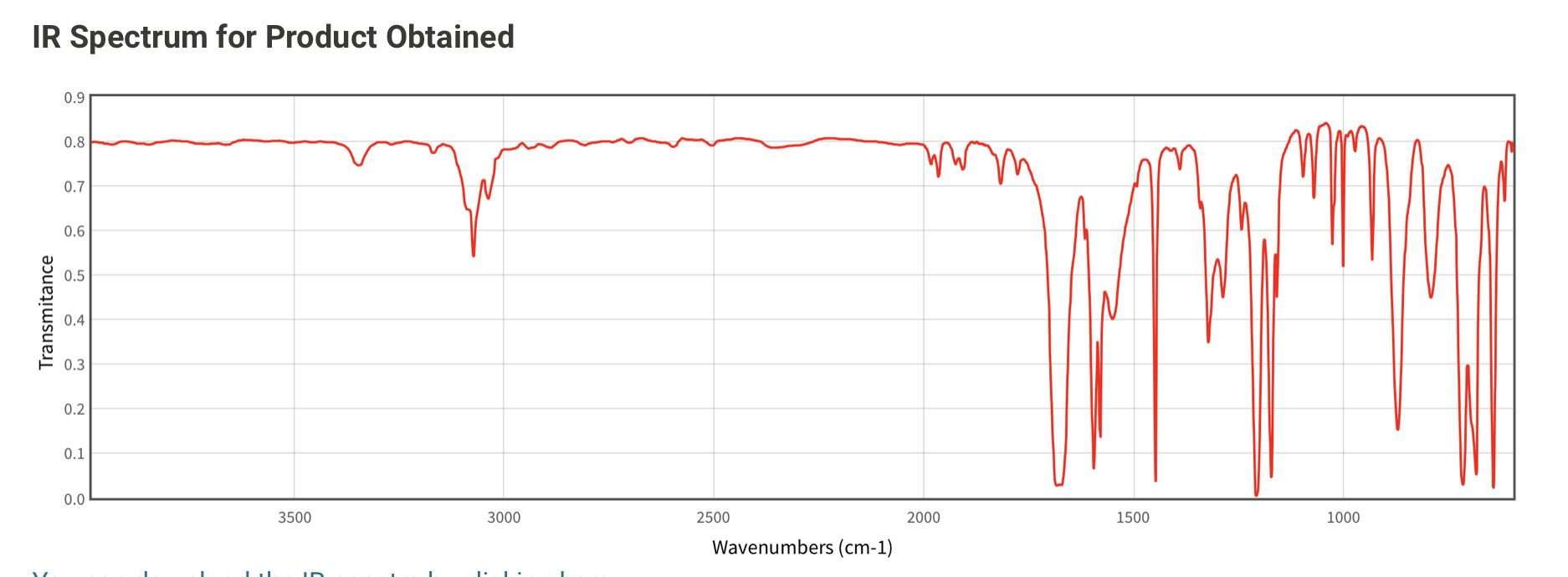
One way to characterize and identify benzoin is through infrared (IR) spectroscopy. IR spectroscopy involves irradiating a sample with infrared light and measuring the absorption of the light by the sample at different wavelengths. The resulting absorption spectrum can be used to identify the functional groups present in the sample and to determine its structural characteristics.
In the IR spectrum of benzoin, the most prominent absorption peaks occur in the following regions:
3000-2800 cm-1: This region corresponds to the stretching vibrations of the O-H bond in the oxo group (-O-). The absorption peak at around 2950 cm-1 is due to the O-H bond in the hydroxyl group (-OH) of benzoin.
1600-1500 cm-1: This region corresponds to the stretching vibrations of the C=O bond in the oxo group (-O-). The absorption peak at around 1650 cm-1 is due to the C=O bond in the aldehyde group (-CHO) of benzoin.
1400-1200 cm-1: This region corresponds to the stretching vibrations of the C-H bonds in the aromatic ring of benzoin. The absorption peak at around 1450 cm-1 is due to the C-H bonds in the para position (i.e., the positions ortho to the oxo group) of the aromatic ring.
1000-700 cm-1: This region corresponds to the stretching vibrations of the C-O bond in the oxo group (-O-). The absorption peak at around 1100 cm-1 is due to the C-O bond in the oxo group of benzoin.
In addition to these absorption peaks, the IR spectrum of benzoin may also show weaker absorption peaks in the region between 4000-3000 cm-1 due to the stretching vibrations of the N-H and O-H bonds, if present.
Overall, the IR spectrum of benzoin can be used to confirm the presence of the oxo group (-O-), the hydroxyl group (-OH), the aldehyde group (-CHO), and the aromatic ring in the compound. It can also provide information about the bond vibrations and the structural characteristics of benzoin, which can be useful in the identification and synthesis of the compound.